Patents
Literature
78 results about "Cathodic polarization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cathodic Polarization. Cathodic Polarization is a corrosion control method by the changing of electrode potential that can be achieved when the potential difference is reduced to a minimum.
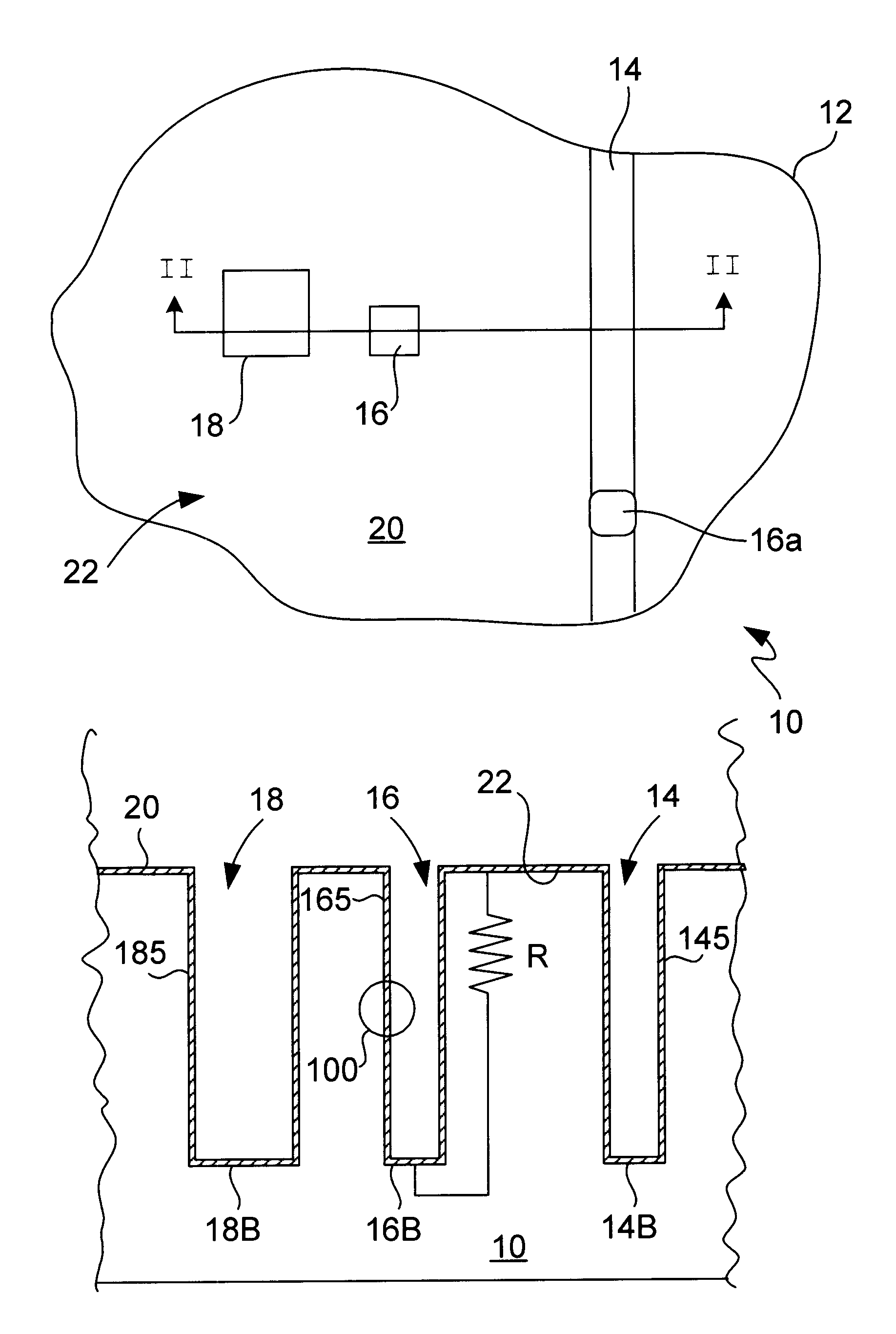
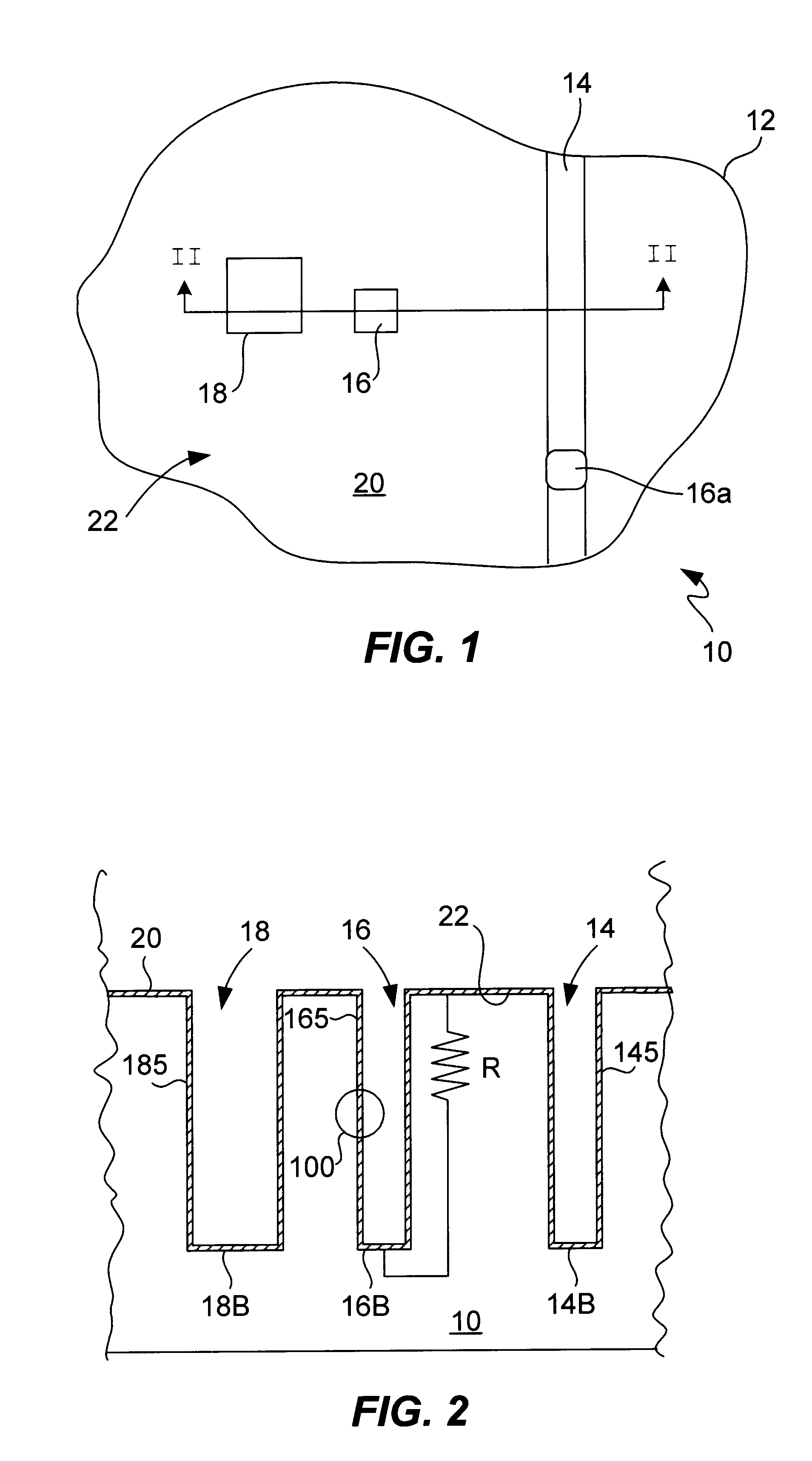
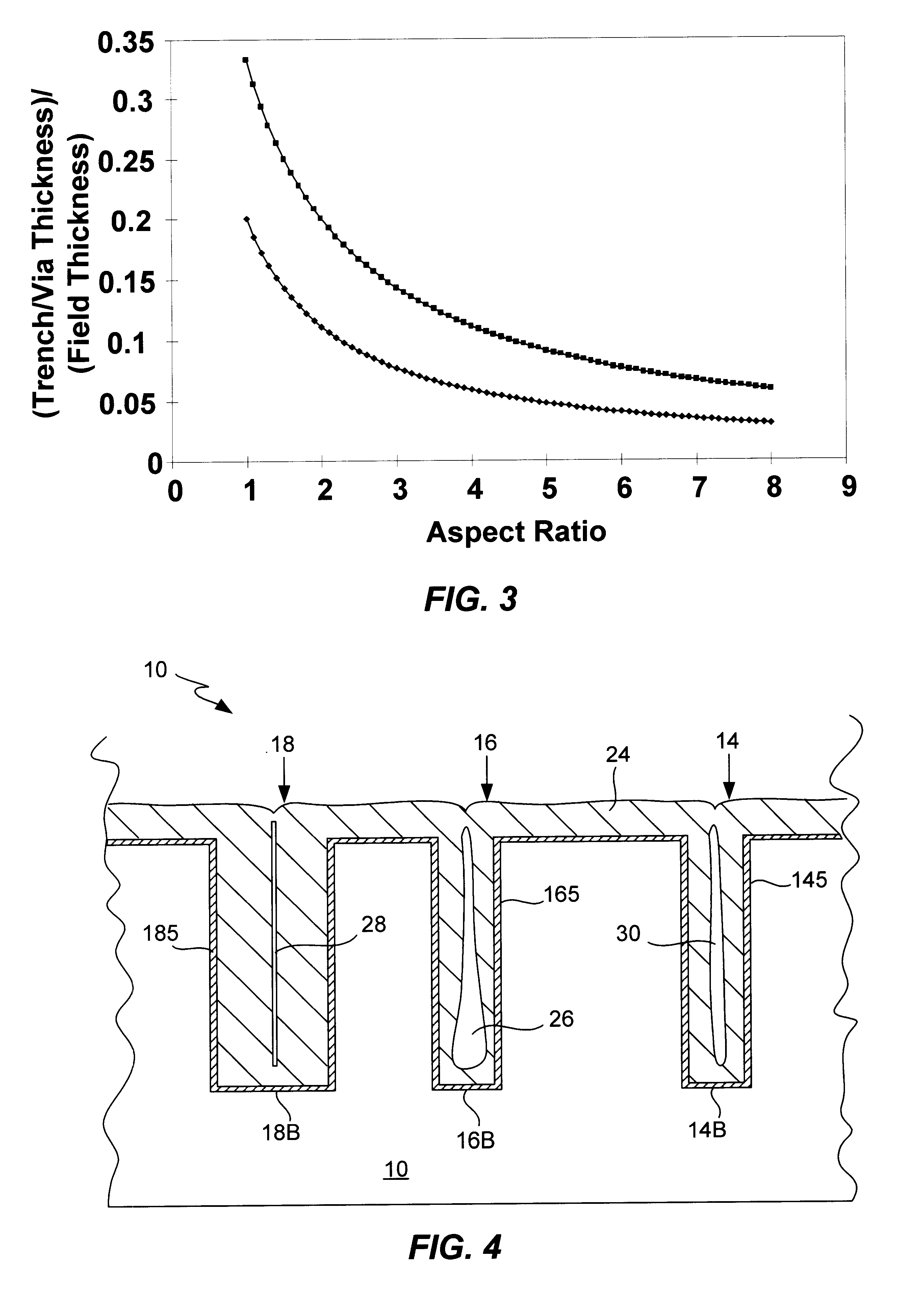
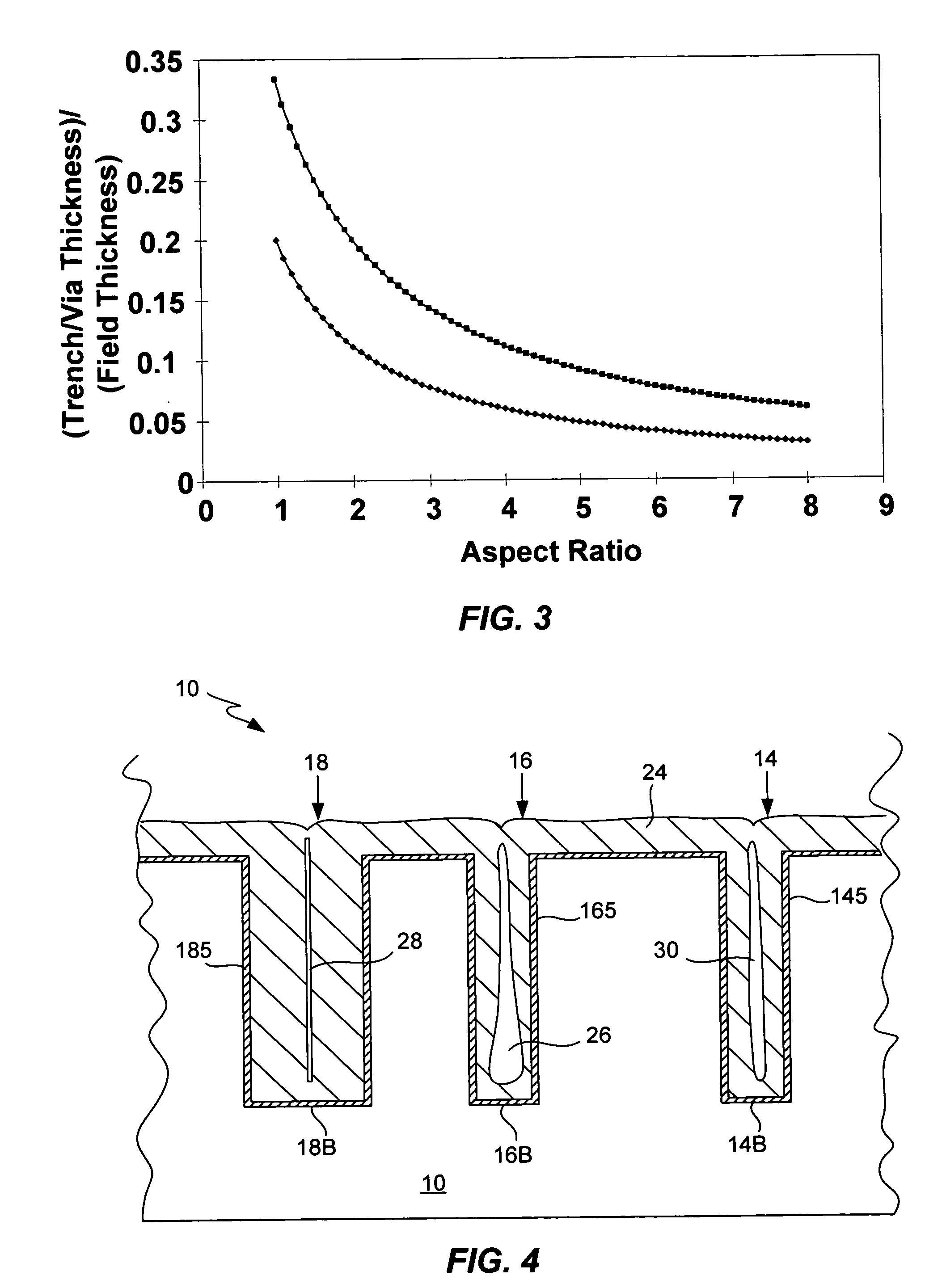
Process for electroplating metal into microscopic recessed features
InactiveUS6946065B1High aspect ratioSmall additive depletion effectAnodisationSemiconductor/solid-state device manufacturingEngineeringCathodic polarization
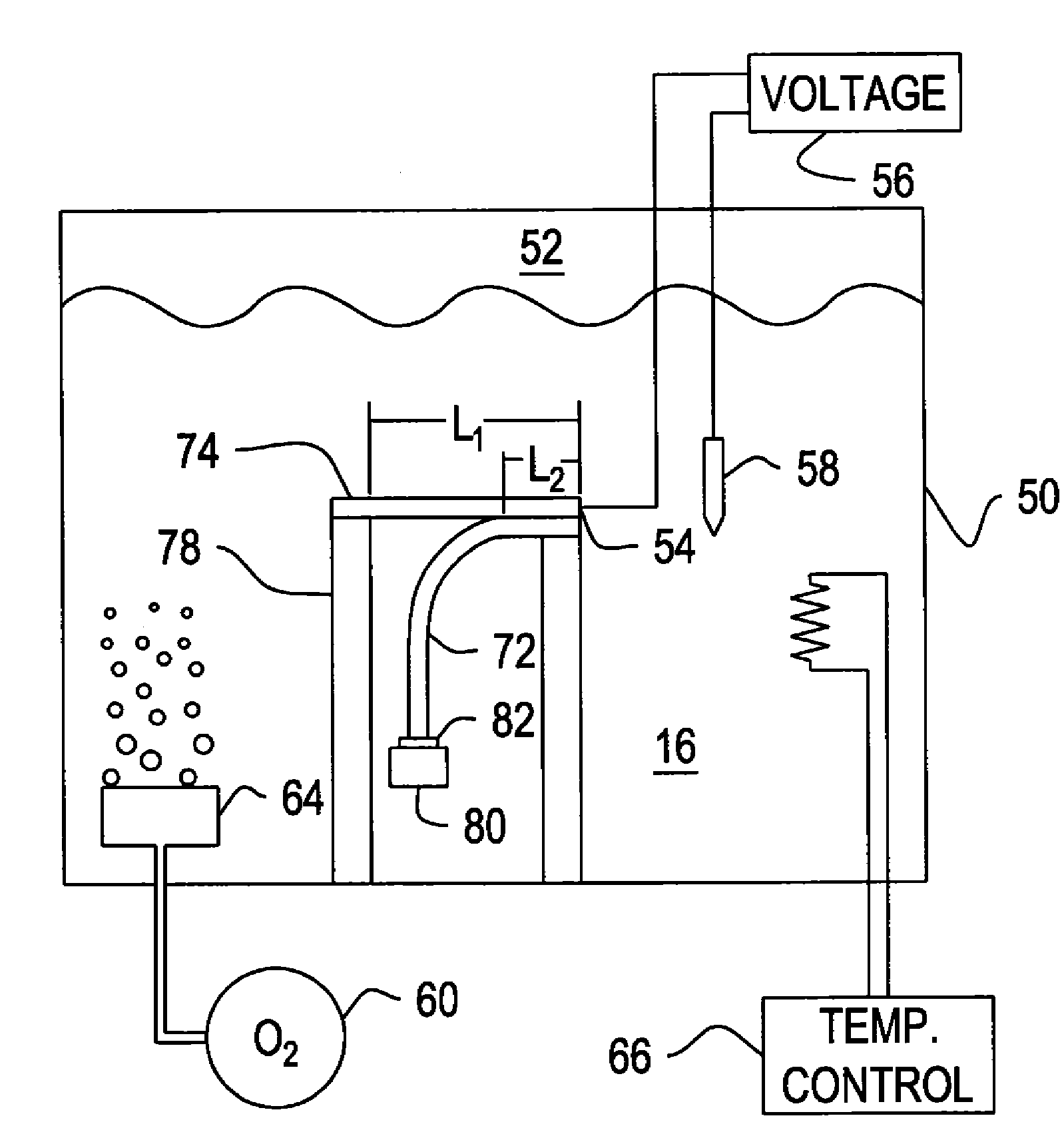
Several techniques are described for reducing or mitigating the formation of seams and / or voids in electroplating the interior regions of microscopic recessed features. Cathodic polarization is used to mitigate the deleterious effects of introducing a substrate plated with a seed layer into an electroplating solution. Also described are diffusion-controlled electroplating techniques to provide for bottom-up filling of trenches and vias, avoiding thereby sidewalls growing together to create seams / voids. A preliminary plating step is also described that plates a thin film of conductor on the interior surfaces of features leading to adequate electrical conductivity to the feature bottom, facilitating bottom-up filling.
Owner:NOVELLUS SYSTEMS
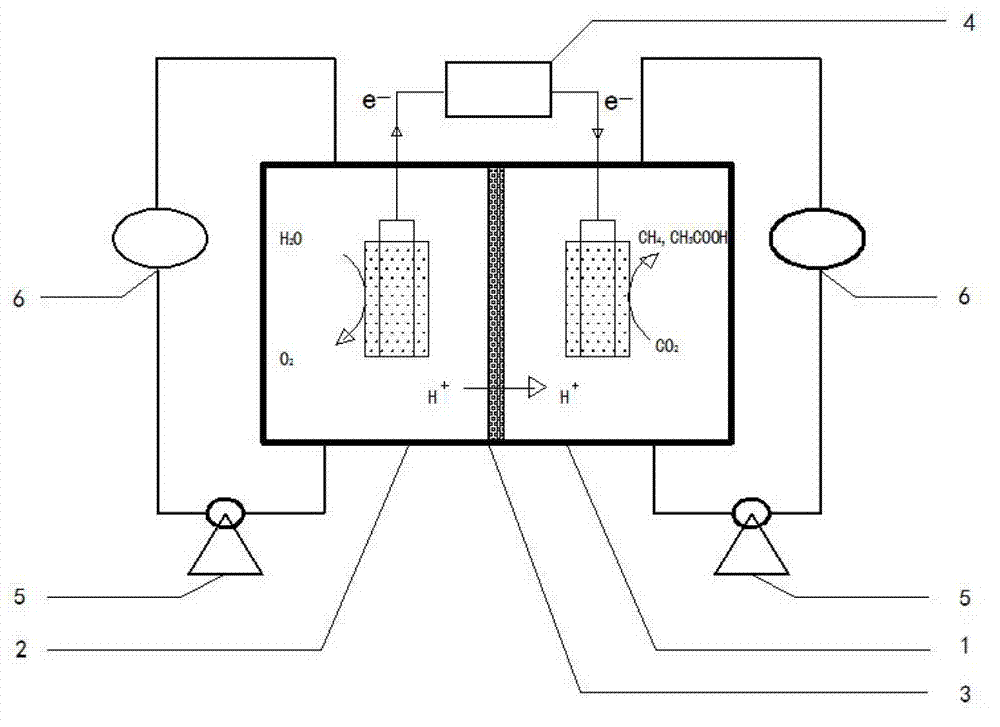
Method for restoring carbon dioxide to produce methane and acetic acid by utilizing biological electrochemical system
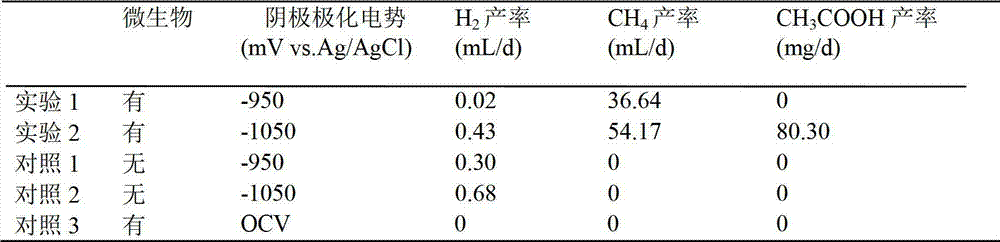
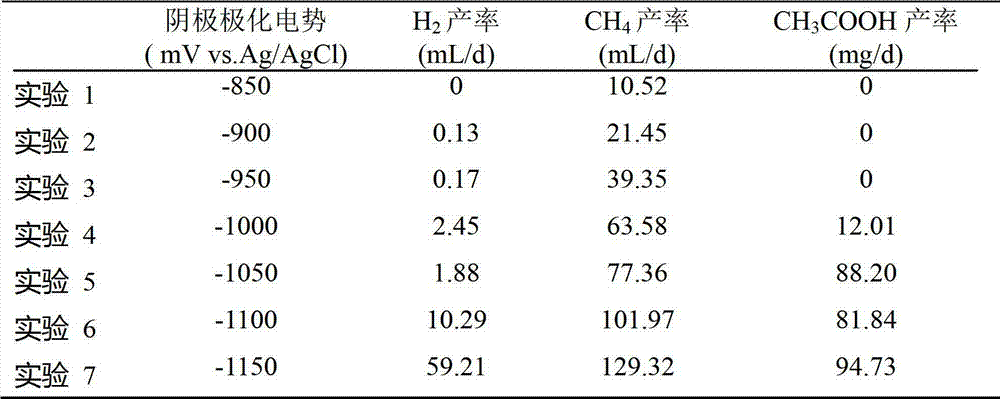
The invention provides a method for restoring carbon dioxide to produce methane and acetic acid by utilizing a biological electrochemical system and regulating and controlling microorganism metabolites by utilizing cathodic polarization potential. A bio-cathode is prepared in the biological electrochemical system, carbon dioxide (CO2) is fed into a cathode chamber and an anode chamber to circularly aerate, the cathodic polarization potential is set from -850mV to -1150mV (vs.Ag / AgCl), and microorganism on a cathode can directly obtain electrons from electrodes or from hydrogen produced by the electrodes to restore the carbon dioxide and produce the methane and the acetic acid. The methane and the acetic acid which are microbial synthesis products can be regulated and controlled by setting different cathodic polarization potentials. The electrodes do not need to use expensive catalyst, and are low in cost. The method for restoring the carbon dioxide to produce the methane and the acetic acid by utilizing the biological electrochemical system is rapid in production rate of the methane and the acetic by restoring the carbon dioxide, and has important application prospect for fixedly converting the carbon dioxide and synthetizing organic chemicals.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Process for electroplating metals into microscopic recessed features
InactiveUS20060011483A1Semiconductor/solid-state device manufacturingSuperimposed coating processElectrical conductorEngineering
Several techniques are described for reducing or mitigating the formation of seams and / or voids in electroplating the interior regions of microscopic recessed features. Cathodic polarization is used to mitigate the deleterious effects of introducing a substrate plated with a seed layer into an electroplating solution. Also described are diffusion-controlled electroplating techniques to provide for bottom-up filling of trenches and vias, avoiding thereby sidewalls growing together to create seams / voids. A preliminary plating step is also described that plates a thin film of conductor on the interior surfaces of features leading to adequate electrical conductivity to the feature bottom, facilitating bottom-up filling.
Owner:NOVELLUS SYSTEMS
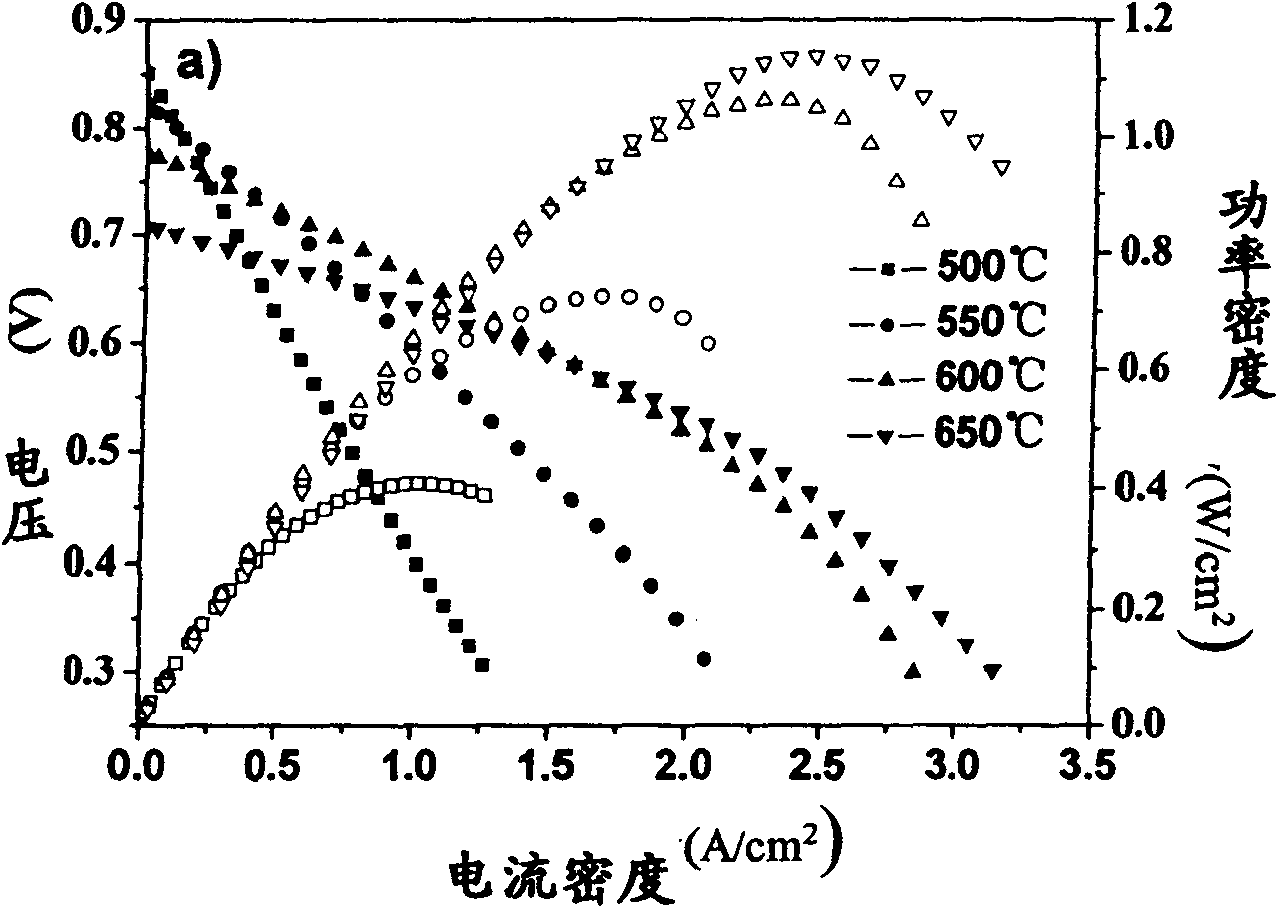
Cathode material of solid oxide fuel cell in A omission type perovskite structure
InactiveCN101847725AEffective control of microstructureImprove battery performanceCell electrodesSolid electrolyte fuel cellsChemical physicsFuel cells
The invention relates to a cathode material of a solid oxide fuel cell, in particular to an A omission Ba1-z(Co1-x-yFexMy)O3-delta(BCFM, x=0 to 0.9, y=0.1 to 0.5, and z=0.01 to 0.3) perovskite cathode material and application thereof, wherein M is Zr, V, Nb, Ti, W, Mo, Ta or Hf. The invention is characterized in that the Ba omission in introduced in a site A on titanium ore type BaCo1-xFexO3-delta(BCF) materials, high-valence ions are doped in a site B, the cobalt content is reduced, and the molecular formula is Ba1-z(Co1-x-yFexMy)O3-delta. The porous A omission site B high-valence doping perovskite materials prepared by the invention have the advantages of good thermal and chemical stability and high oxygen catalytic activity, and can effectively reduce the cathodic polarization. The BCFM materials singly form an SOFC cathode or form the SOFC cathode with electrolyte materials through being compounded. The cathode is in a nano-micro structure with two layers, wherein the outer layer is in a large-grain loose structure, and the inner layer is in a fine-grain relatively compact structure. The BCFM cathode can be used as the SOFC cathodes with different structures and compositions. The output power of the cell at 650 to 700 DEG C is about 1.0 W / cm<2>.
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
Preparation method of titanium alloy surface passivation film transmission observation sample
InactiveCN103364243AEvenly distributedFully distributedPreparing sample for investigationSand-paperTitanium
The invention relates to the field of preparation of a transmission sample, and in particular to a preparation method of a titanium alloy surface passivation film transmission observation sample. The method comprises the following steps of: (1) performing double-spray thinning treatment on a transmission sample with diameter of 3mm and thickness of 40-50 microns polished by sand paper in acid liquor at -20to -30 DEG C to prepare a transmission sample with thin region thickness of about 30nm-100nm and uniform distribution; (2) performing cathodic polarization on the transmission sample subjected to the double spray in electrolyte under the condition of -0.5V to -1.5V for about 1-10 min so as to remove an oxide film spontaneously formed on the surface of the sample in air; and (3) performing anodic passivation treatment on the alloy sample under the condition of 0 to 2V for 0.5-5 hours to fully oxidize the alloy surface so as to form a passivation film uniformly distributed at the thin region of the transmission sample. The fully passivated thin region is completely made from metal oxide and can be free from the influence of an alloy phase of a matrix, and thus the phase composition and the element distribution of the passivation film are easily observed. The preparation method is suitable for preparing of the transmission observation sample of the passivation film of titanium, titanium alloy and the like with thickness of only several nanometers.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Preparation method of cathode of low-medium temperature solid oxide fuel cell
InactiveCN102104153ASuppress coarseningOptimal Control StructureCell electrodesConcentration polarizationFuel cells
The invention relates to a preparation method of a cathode of a low-medium temperature solid oxide fuel cell, belonging to the technical field of fuel cells. The preparation method comprises the following steps of: preparing a cathode layer chief source by spraying cathode slurry on an electrolytic substrate and drying at the temperature of 100-400 DEG C; and then sintering at 800-1300 DEG C in an air environment for 2-10 hours so as to obtain the cathode of the fuel cell, wherein temperature rise rate is 5-20 DEG C / minute. The invention solves the problems of overhigh oxygenic concentration polarization and higher cathodic polarization impedance which are caused by difficult structure control, poor preparation repeatability and slower oxygenic cathodic transmission of a cathode layer of the prior common low-medium temperature solid oxide fuel cell and prepares the cathode layer which has specific lamellar structure, needs no template and can be prepared easily and repeatedly by selecting a powder synthesis process, calcining temperature and temperature rise rate, thereby obviously enhancing the cathode performance.
Owner:SHANGHAI JIAO TONG UNIV
Method for forming corrosion inhibiting film on steel and iron surface
InactiveCN102352501ASimple methodSimple and fast operationElectrolytic coatingsMetallic material coating processesRoom temperatureCathodic polarization
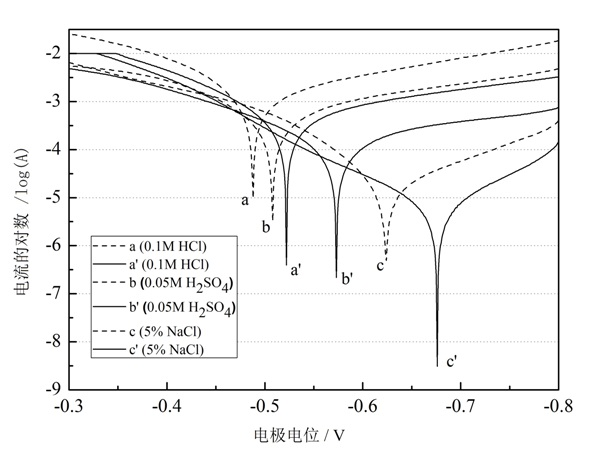
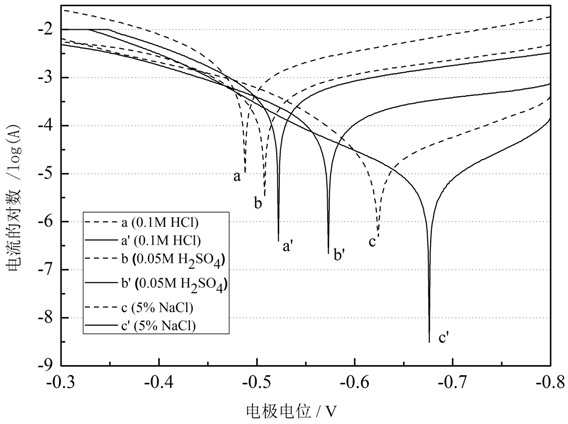
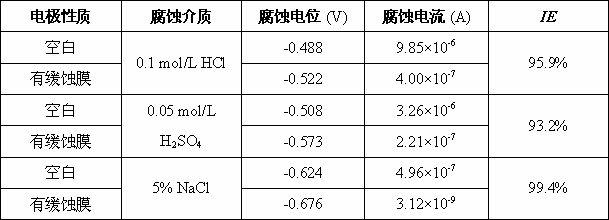
The invention relates to a method for forming a corrosion inhibiting film on a steel and iron surface, which comprises the following steps that: firstly, a steel and iron sheet is gradually ground and polished by No. 1# to 6# metallographic abrasive paper grade by grade, is cleanly washed by deionized water, is degreased by acetone and is cleanly washed by the deionized water, an oxidation film is removed through dilute sulphuric acid, and the steel and iron sheet is cleanly washed by the deionized water for use; self assembly solution is formed by diazonium salt solution with the concentration being 0.005mol / L to 0.02mol / L and dilute sulphuric acid solution with the concentration being 0.01 to 0.1mol / L; the steel and iron sheet is soaked into the mixed self assembly solution, the soakingtemperature is the room temperature, and the assembly time is 1 to 3h; cathodic polarization is carried out on electrode in the mixed solution (at the potential range being -0.5 to -1.5V), and the time is 200 to 400s. The method has the beneficial effects that the method for forming the corrosion inhibiting film on the surface of the iron and steel electrode is simple, the operation is simple andconvenient, and the corrosion inhibiting effect is excellent. Electrochemical data shows that the corrosion inhibiting efficiency of the corrosion inhibiting film in corrosion media of 0.1mol / l HCl, 0.05mol / L H2SO4 and 5 percent NaCl is respectively higher than 90 percent.
Owner:SHANGHAI UNIV
Method for preparing electrodeposited cobalt under large flow
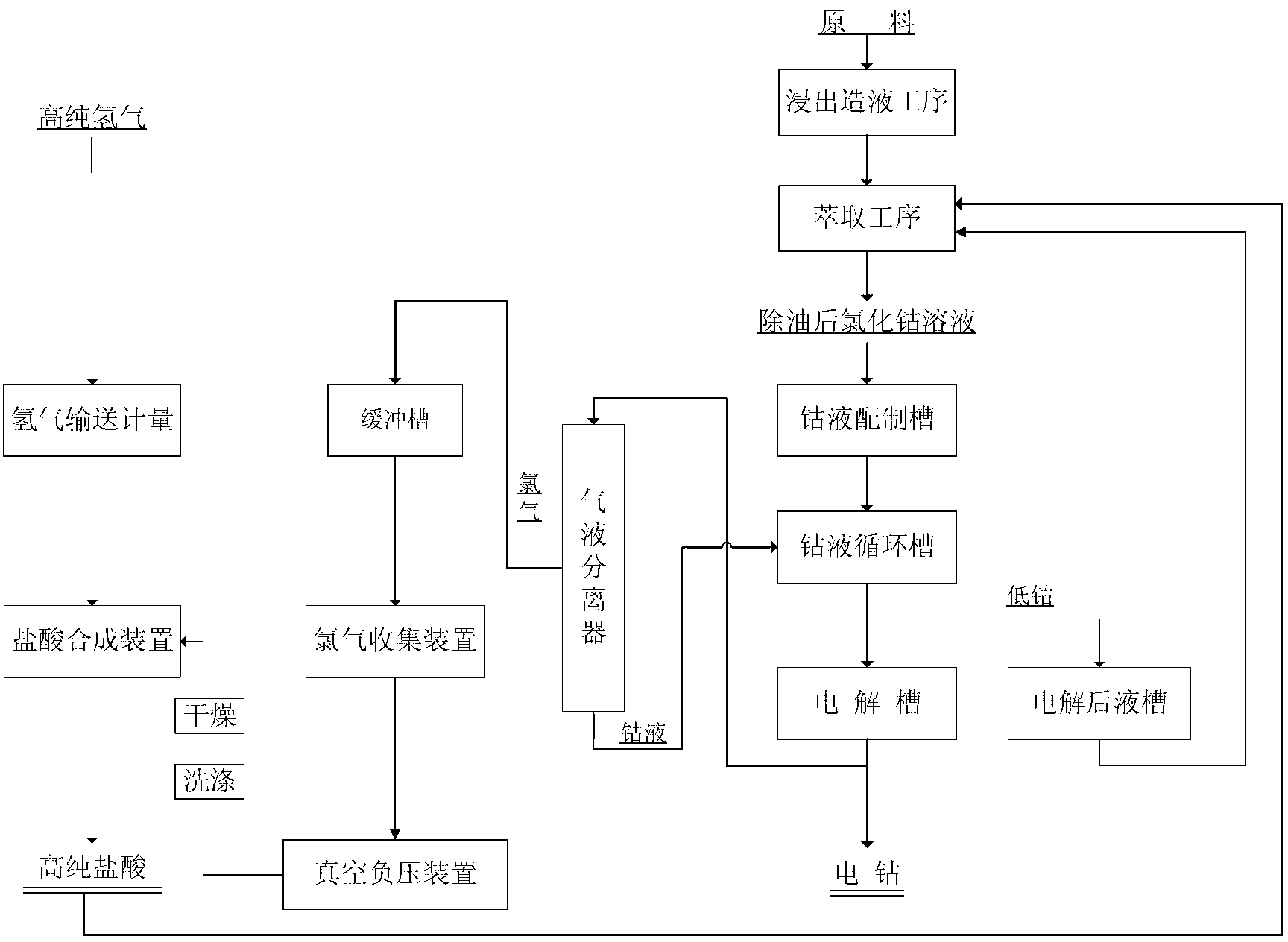
ActiveCN103060842AImprove current efficiencyReduce cathodic polarizationPhotography auxillary processesWater chlorinationHydrometallurgy
The invention discloses a method for preparing electrodeposited cobalt under a large flow. In conventional production methods for electrodeposited cobalt, the process of processing has the disadvantages of low current efficiency and long electrodeposition time, and purity and a recovery rate of produced electrodeposited cobalt hardly reach requirements. A technical scheme employed in the invention is as follows: a deoiled cobalt chloride solution produced in wet metallurgy is used as a raw material and is diluted with pure water so as to allow the concentration of Co<2+> in the cobalt chloride solution to be 30 to 100 g / L, and the diluted cobalt chloride solution is used as electrodeposition pre-liquid; and electrodeposition is carried out on the electrodeposition pre-liquid in an enclosed electrodeposition apparatus under the conditions of a large flow of 5 to 15 m<3> / h.m<2> and a negative pressure. According to the invention, the large flow is employed in the process of preparation of electrodeposited cobalt, so cathodic polarization is reduced, high current efficiency as high as more than 95% is obtained, and direct current consumption is low; utilization of the large flow enables chlorine and oxygen generated by an anode to be taken away in time, and oxidation of the cathode cobalt by chlorine and oxygen is prevented, thereby guaranteeing product quality.
Owner:ZHEJIANG HUAYOU COBALT +1
Ionic liquid gold plating solution containing coordination agent and additive and gold plating method adopting ionic liquid gold plating solution
The invention discloses an ionic liquid gold plating solution containing a coordination agent and an additive and a gold plating method adopting the ionic liquid gold plating solution and belongs to the technical field of gold electroplating. The gold plating solution is prepared from a solvent [BMIm] [BF4], main salt chloroauric acid, the coordination agent and the additive. Gold electroplating mainly includes the steps of (1) a substrate pretreatment and nickel preplating process and (2) gold electroplating. The ionic liquid gold plating solution containing the coordination agent and the additive and the gold plating method adopting the ionic liquid gold plating solution have the advantages that the added coordination agent has a certain coordination relation to gold ions, the stability of a gold complex in the plating solution can be improved, cathodic polarization can be improved in the electroplating process, and the functions of refining grains and brightening plating coatings are achieved; the added additive cooperates with the coordination agent to a certain degree so that the grains can be further refined, the coatings can be more compact and the appearance can be brighter; and to sum up, the ionic liquid gold plating solution and the gold plating method have the best characteristic that by adding the coordination agent and the additive in an ionic liquid system, the stability of the gold complex in the gold plating solution and the stability of the gold plating solution are improved, and therefore the gold plating coatings and the gold plating solution which are good in properties are obtained.
Owner:HARBIN INST OF TECH
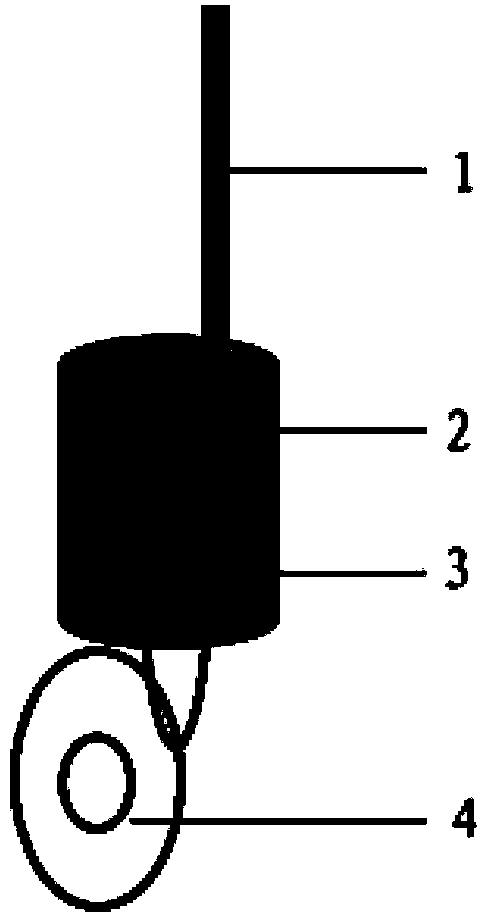
Nickel positive electrode for fiber battery
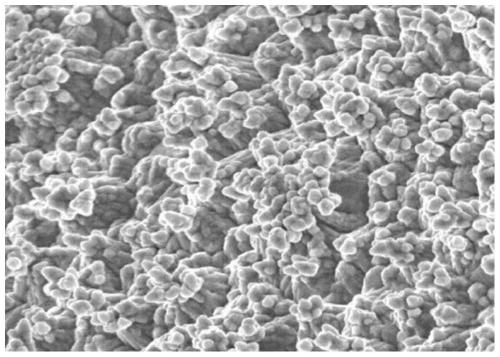
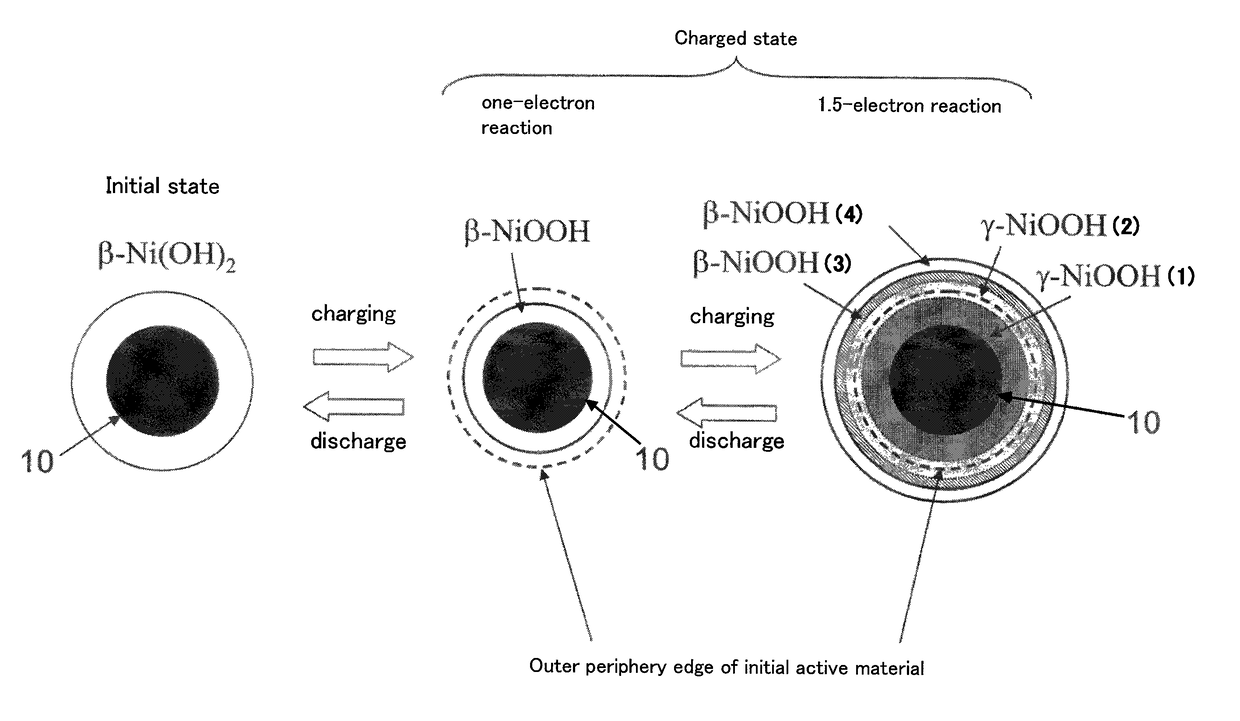
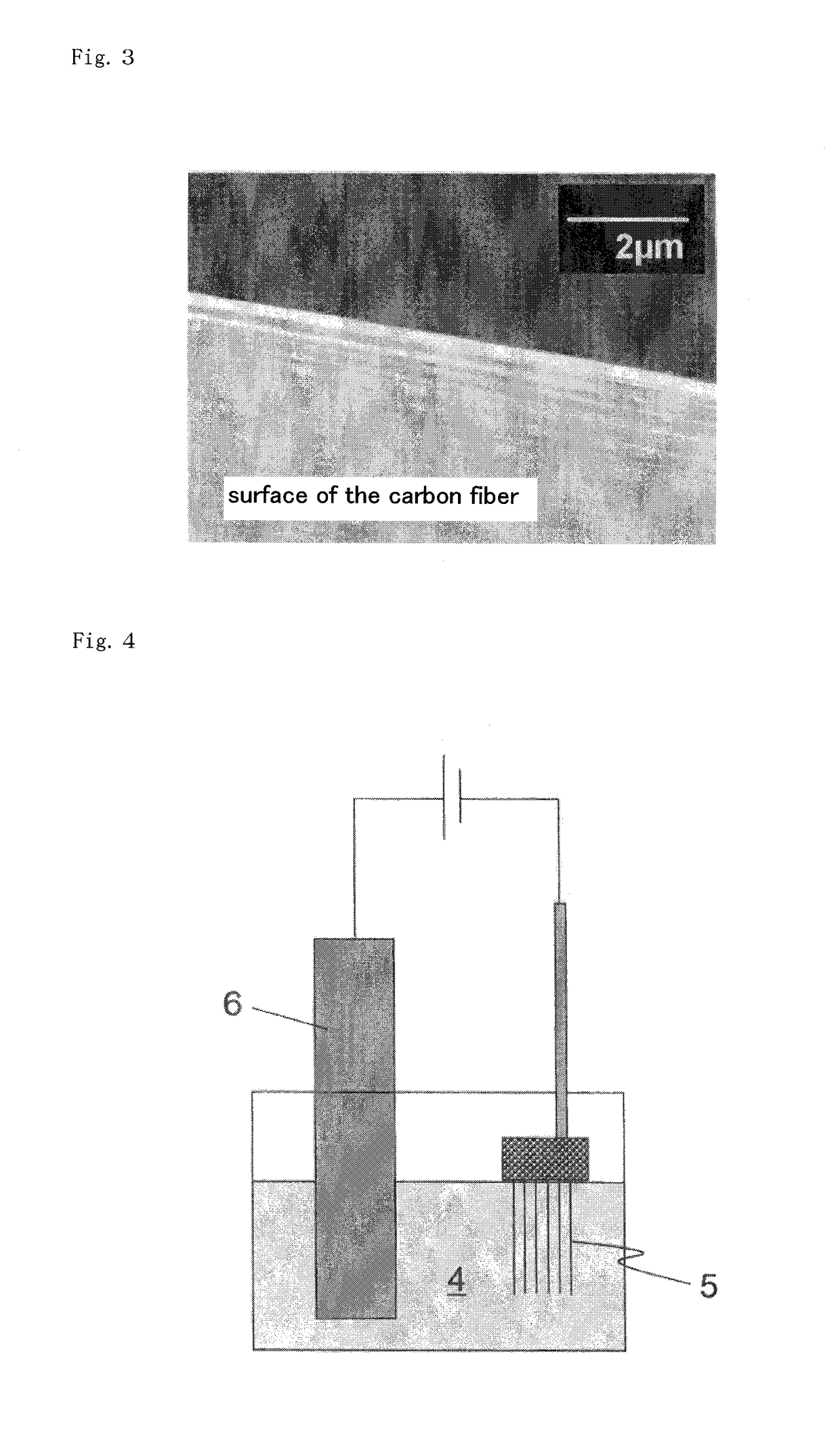
InactiveCN102217122ASolution to short lifeHigh outputElectrode manufacturing processesElectrode carriers/collectorsFiberNitrate
Owner:KAWASAKI JUKOGYO KK
Measuring method of deep-hole copper plating accelerant
ActiveCN103698384AEase of industrial applicationMaterial electrochemical variablesCopper platingProcess quality
The invention relates to a measuring method of a deep-hole copper plating accelerant. The measuring method comprises the following steps: (1) forming a three-electrode system by a metal electrode, an inert metal sheet and a saturated calomel electrode which are taken as a working electrode, a counter electrode and a reference electrode respectively, and selecting a copper electroplating solution; (2) measuring a cathodic polarization curve of an accelerant-free basic electroplating solution; (3) adding accelerants of different concentration into the basic electroplating solution, and respectively measuring corresponding cathodic polarization curves; (4) comparing the cathodic polarization curve of the step (2) with the cathodic polarization curves of the step (3), and analyzing relevant parameters of the curves so as to acquire a quantitative relation curve between relevant electrochemical parameters and the concentration of the accelerants; and (5) measuring an actual to-be-measured copper plating solution by the same electrode system so as to obtain a result. By taking the cathodic polarization curves as measuring objects, the measuring method is close to an actual electroplating process, so that the management and process quality control of the copper electroplating solution are directed, and the industrial application of a TSV copper electroplating process is promoted.
Owner:SHANGHAI JIAO TONG UNIV
Cathodic corrosion protection powder coating composition and method
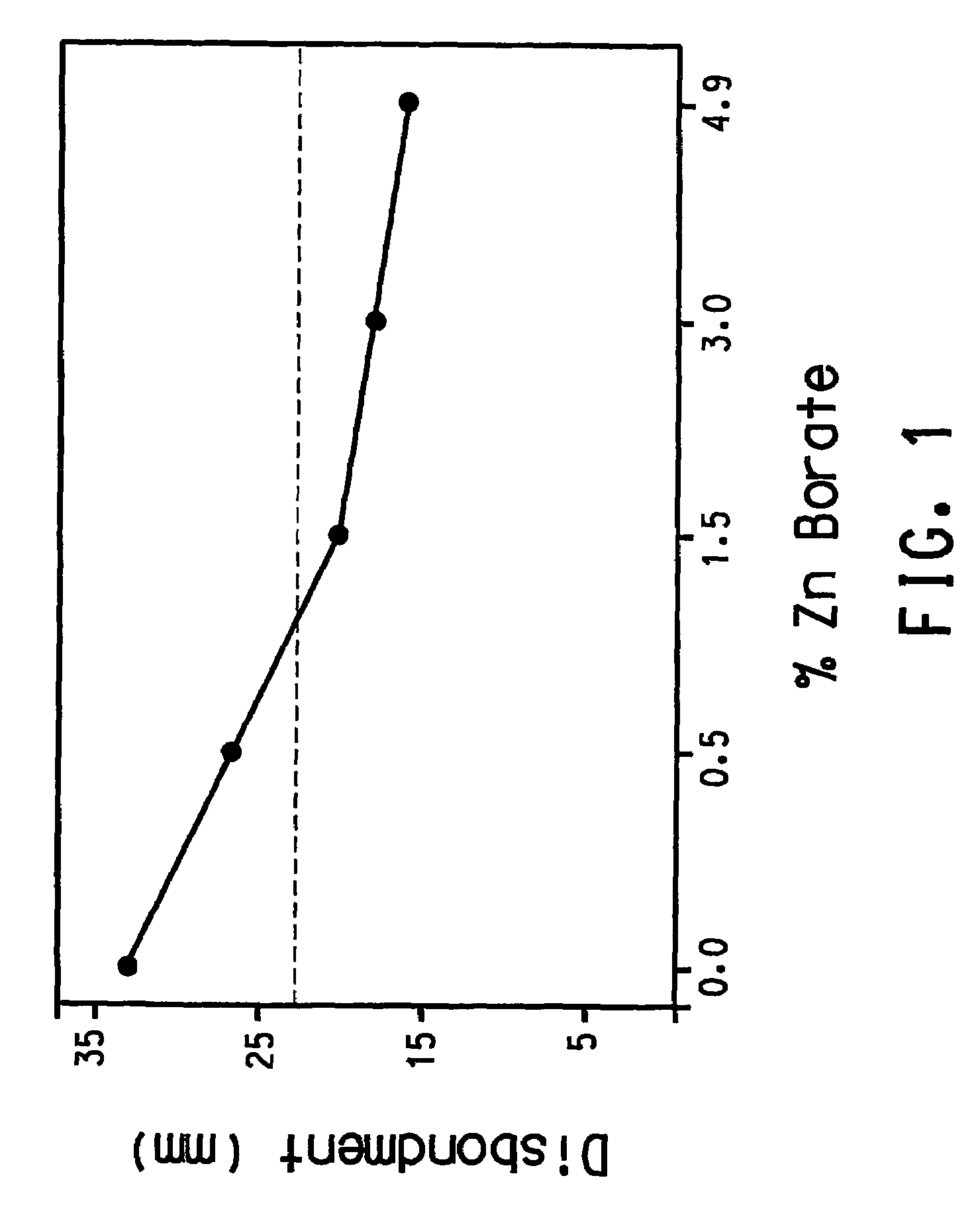
The present invention provides a curable cathodic corrosion protection powder coating, which comprises a thermosetting resin, a zinc borate compound, a curing agent in an amount effective to cure the coating. Further, the present invention also provides a method of cathode corrosion protection which includes the steps of subjecting the substrate to a mechanical treatment, applying to said treated steel surface, the cathodic protective coating, and polarizing the coated material as a cathode.
Owner:AXALTA COATING SYST IP CO LLC
Nickel Positive Electrode for Fiber Battery
ActiveUS20110287320A1Increase productionLong life durationElectrode manufacturing processesElectrode carriers/collectorsFiberCarbon fibers
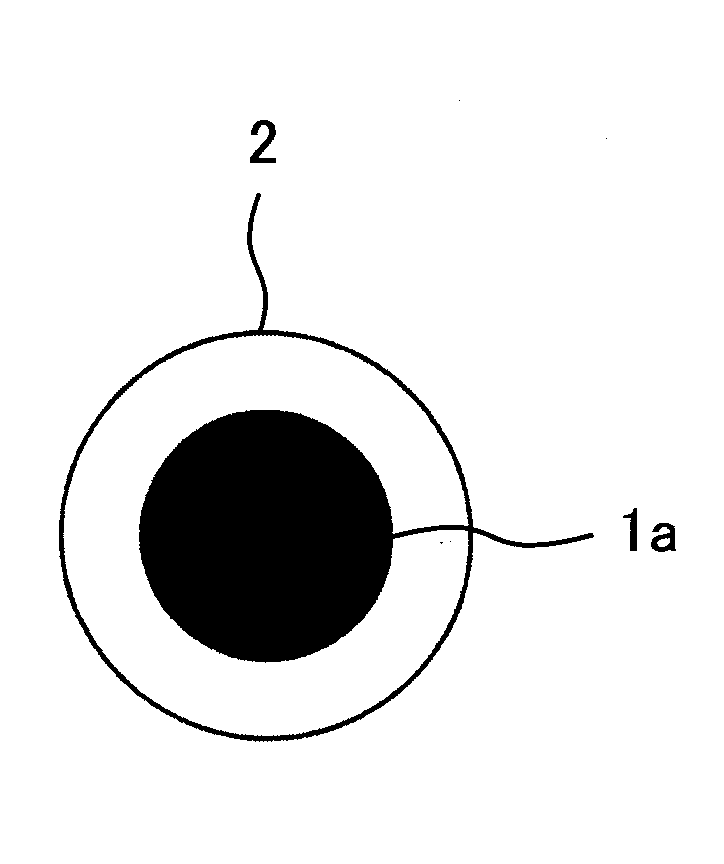
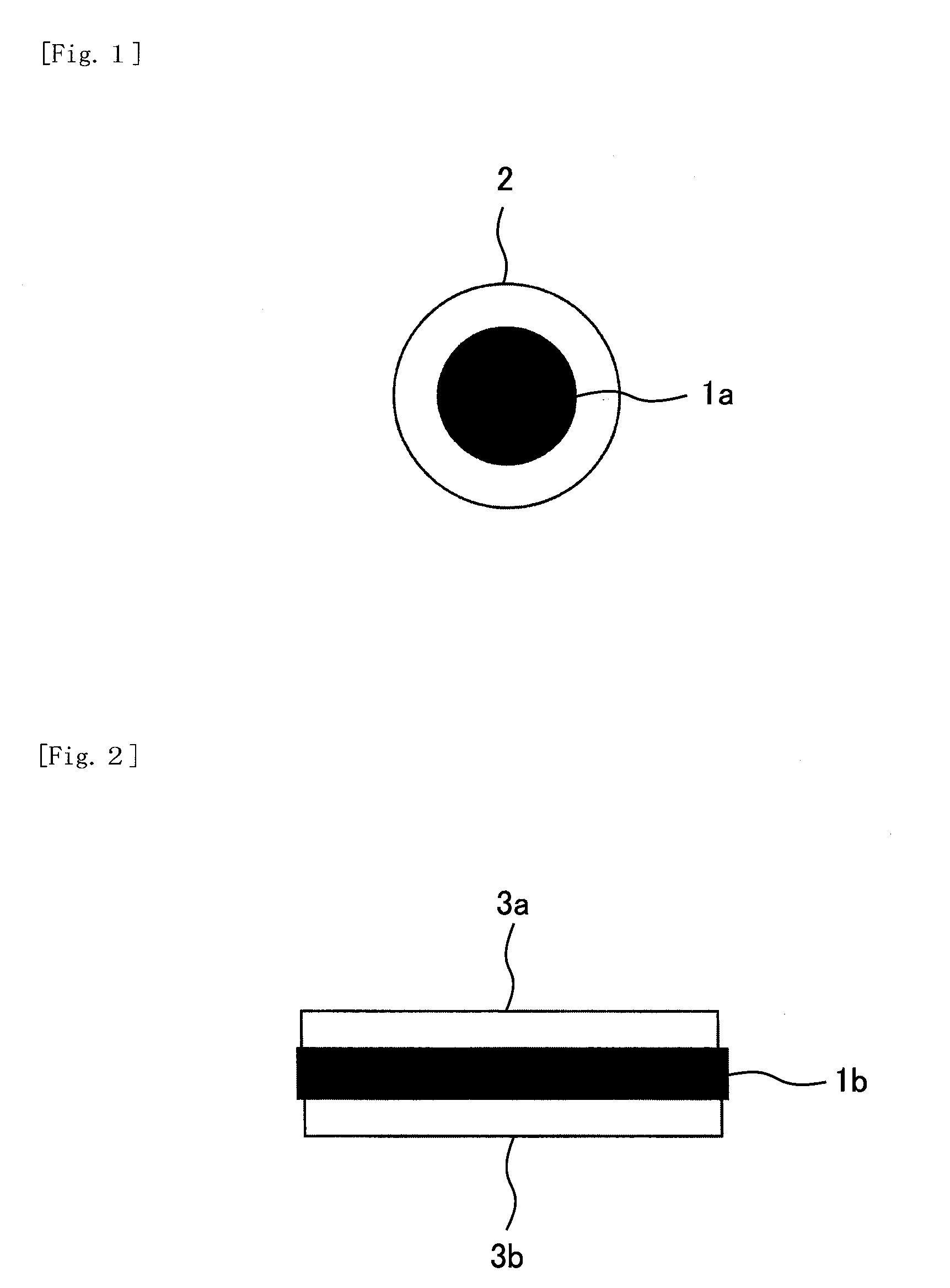
Disclosed is a nickel positive electrode for a fiber battery having a long life duration, and also being enabling a high output and high capacity to be attained. For this purpose, the nickel positive electrode for a fiber battery is obtained by coating a carbon fiber with nickel, then causing a cathodic polarization in a nickel nitrate bath using the nickel-coated carbon fiber as a cathode, and then immersing the precipitate, which was deposited on the surface of the carbon fiber by the cathodic polarization, in an aqueous caustic alkali solution.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
Method for preparing catelectrode material LnA'CuO with K2NiF4 structure by electrostatic spinning
InactiveCN101572315APolarization resistance is smallSmall cathodic polarization potentialCell electrodesFilament/thread formingCathodic polarizationChemical stability
The invention provides a method for preparing catelectrode material LnA'CuO with a K2NiF4 structure by electrostatic spinning, which relates to a method for preparing the catelectrode material. The invention solves the problems of poor chemical stability and poor electrode performance of the existing catelectrode material. The method is as follows: a precursor solution of LnA'CuO and an ethanol solution of polyvinylpyrrolidone are stirred and mixed to stand still; obtained collosol is filled in a syringe, is spun for 6h under the injector pump conditions of 5m1 / h flow rate and 30 kV voltage, and then is dried to be treated by double sintering so as to obtain the catelectrode material LnA'CuO.The catelectrode material LnA'CuO obtained in the invention is fibrous, and the diameter is 200 to 350nm; the value of polarization resistance is low, and the value of specific conductivity is 10 to 40 Scm; cathodic polarization overpotential is less than SrSmNiO4 and La0.8Sr0.2Co0.4Fe0.6O3.
Owner:HEILONGJIANG UNIV
Coated Metallic Sample Peel Test
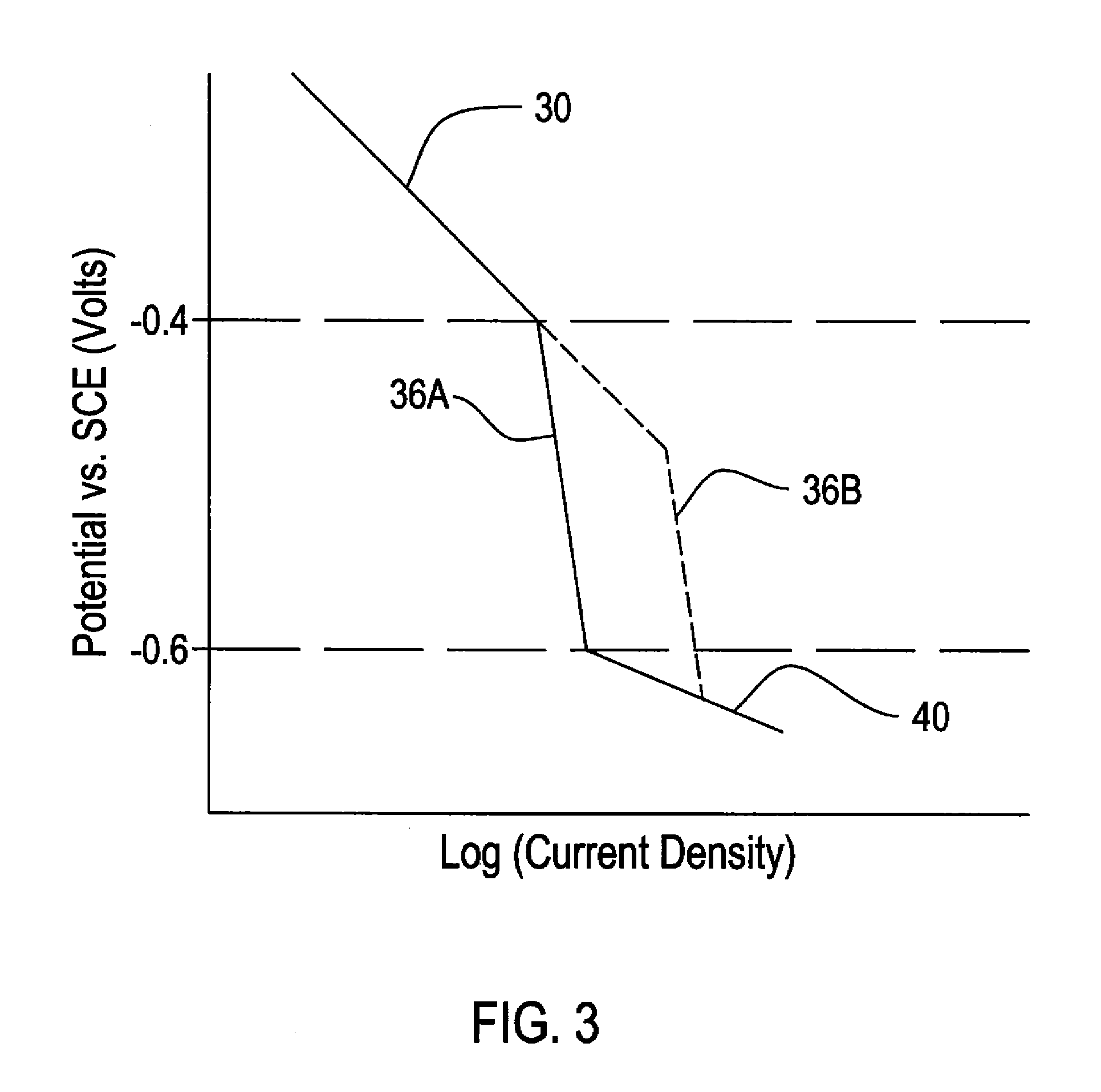
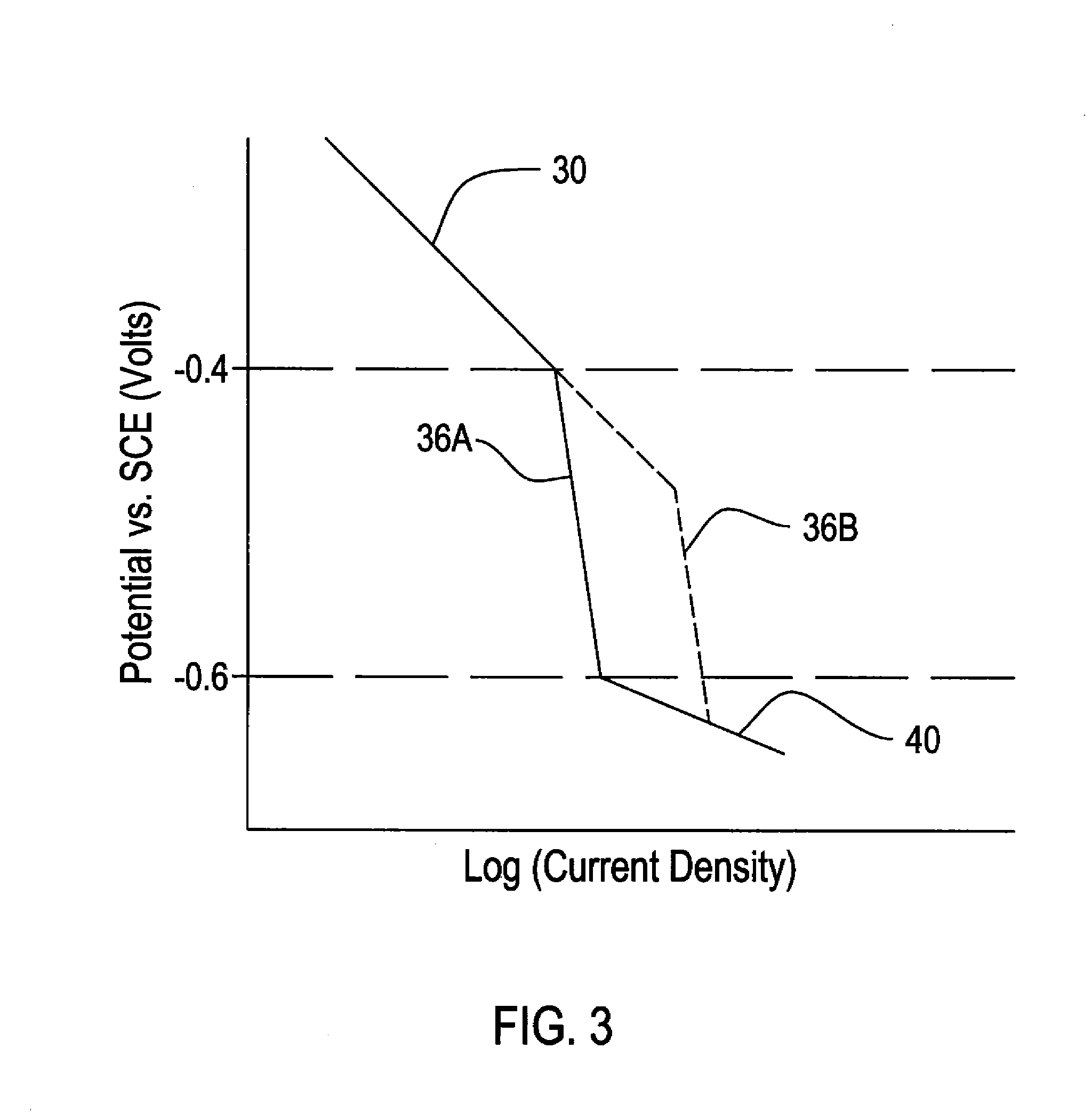
A method for conducting an accelerated life test of a polymer coated metallic sample includes placing the sample below the water surface in a test tank containing water and an oxygen containing gas. Cathodic polarization of the metallic portion of the sample is increased. This can be by using a voltage source or a sacrificial anode. Dissolved oxygen in the test tank water is also increased. Dissolved oxygen can be increased by providing oxygen under pressure to the tank or through an aerator under the water surface. Temperature can also be regulated to accelerate the test speed. Delamination of the sample is periodically tested through a peel test or by other means. The invention also provides a reaction model independent method for calculating activation energy.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Cathodic delamination accelerated life test method
InactiveUS20090026093A1Increase probabilityWeather/light/corrosion resistanceVolume/mass flow measurementActivation energyVoltage source
A method for conducting an accelerated life test of a polymer coated metallic sample includes placing the sample below the water surface in a test tank containing water and an oxygen containing gas. Cathodic polarization of the metallic portion of the sample is increased. This can be by using a voltage source or a sacrificial anode. Dissolved oxygen in the test tank water is also increased. Dissolved oxygen can be increased by providing oxygen under pressure to the tank or through an aerator under the water surface. Temperature can also be regulated to accelerate the test speed. Delamination of the sample is periodically tested through a peel test or by other means. The invention also provides a reaction model independent method for calculating activation energy.
Owner:UNITED STATES OF AMERICA NAVAL UNDERSEA WARFARE CENT DIV NEWPORT OFFICE OF COUNSEL THE
Preparation method of nano-crystalline zinc plating layer by using direct current electrodeposition
The invention belongs to the technical field of surface engineering and surface treatment, in particular providing a preparation method of a nano-crystalline zinc plating layer by using direct current electrodeposition. The preparation method is characterized by taking acidic zinc sulfate solution as a base plating solution, adopting three organics of cetyl trimethyl ammonium bromide (CTAB), benzylidene acetone (BA) and polyethylene glycol (PEG) as mixed additives for remarkably increasing the cathodic polarization of zinc deposition; and applying direct current for deposition by taking carbon plated steel as a cathode and insoluble electrode as an anode with the application of the direct current electrodeposition method, wherein current density is 0.5-4 A / cm2, and electroplating time is 1-6 minutes. By adopting the invention, the zinc plating layer with a luminant and smooth surface and the size of a crystalline grain less than 50 nm.
Owner:SHANGHAI UNIV
Preparation method of porous titanium-substrate-loaded nickel oxide (nickel hydroxide) electrode with electroconductive ceramic interface
InactiveCN108281291AImprove corrosion resistancePorosityHybrid capacitor electrodesHybrid/EDL manufactureCathodic polarizationContact resistance
The invention discloses a preparation method of a porous titanium-substrate-loaded nickel oxide (nickel hydroxide) electrode with an electroconductive ceramic interface. The preparation method includes (1), ball-milling and mixing metal titanium hydride powder and nickel powder to obtain a metal powder mixture;(2), putting a certain amount of the metal powder mixture into a steel die, and performing pressurization to obtain a metal pressed blank; (3), putting the metal pressed blank into a tubular furnace, and controlling sintering atmosphere and temperature time to obtain the porous titanium-substrate-loaded nickel oxide electrode with the electroconductive ceramic interface; (4), washing the surface of the electrode by dilute acid and deionized water; (5), in nickel nitrate, depositing acertain amount of nickel hydroxide on the surface of the electrode according to a cathodic polarization method to obtain the porous titanium-substrate-loaded nickel hydroxide electrode with the electroconductive ceramic interface. The preparation method has the advantages that the porous electroconductive ceramic interface TinO2n-1-Ti*NiOy generated during high-temperature hypoxic sintering is utilized, contact resistance between active substances and a substrate is reduced, and contact strength between the active substances and the substrate is improved.
Owner:陈军
Iron-nickel alloy in-situ desolventized layered perovskite cathode material for CO2 electrolysis
ActiveCN111394748AIncreased concentration of oxygen vacanciesEnhanced chemical adsorption capacityMaterial nanotechnologyTransportation and packagingCathodic polarizationMaterials science
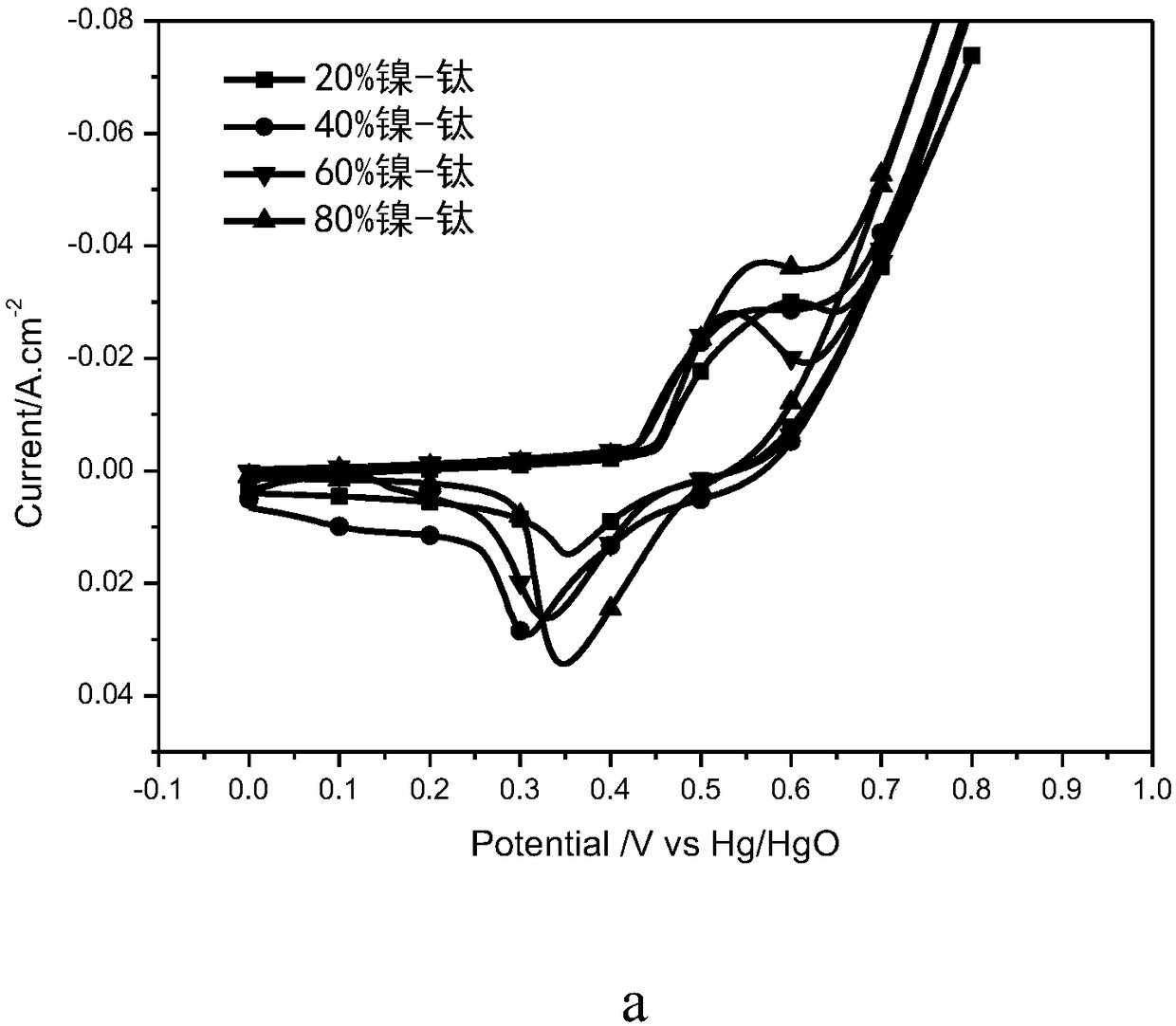
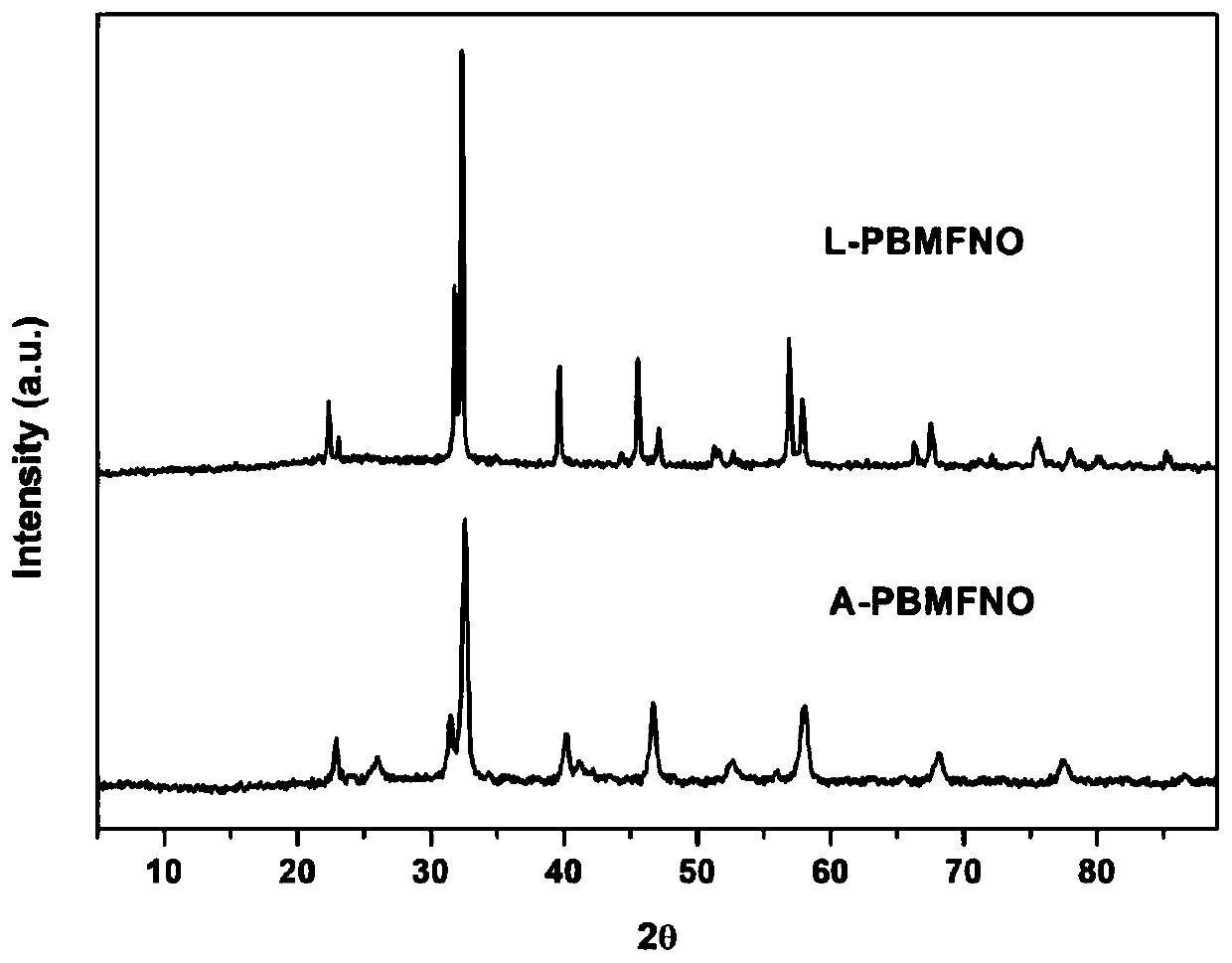
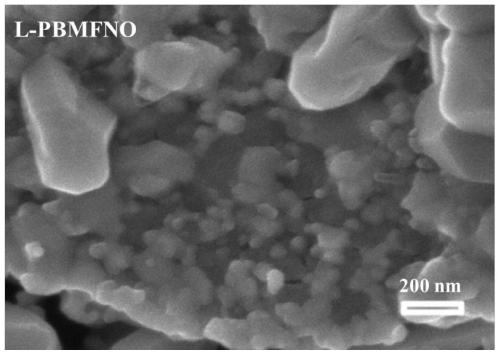
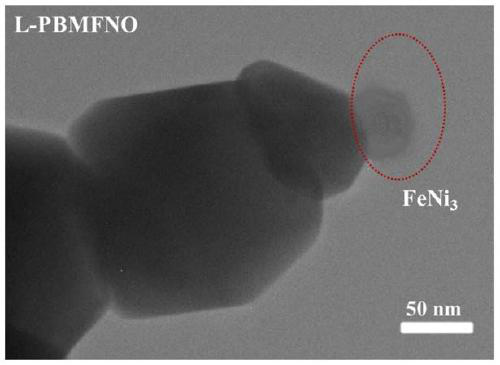
The invention relates to a solid oxide electrolytic cell cathode material for CO2 electrolysis, in particular to an A-vacancy layered perovskite cathode material Pr0.9Ba0.9Mn<2-5x>Fe<4x>Ni<x>O<5+delta> (PBMFNO, x = 0.04-0.20) and an application thereof. The cathode material is characterized in that: Pr and Ba vacancies are introduced into an A site of a titanium ore material Pr0.5Ba0.5MnO3, two transition metal ions including Fe and Ni are doped into a B site, the molecular formula is Pr0.45Ba0.45Mn<1-5y>Fe<4y>Ni<y>O<3-delta> (y is equal to 0.02-0.10), Pr0.45Ba0.45Mn<1-5y>Fe<4y>Ni<y>O<3-delta>is converted into PBMFNO through H2 reduction, and meanwhile, iron-nickel alloy FeNi3 nanoparticles with the size of 30-50 nm are obtained through in-situ desolvation. The perovskite cathode materialprepared by the invention has good stability and CO2 catalytic activity, and can effectively reduce cathode polarization.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
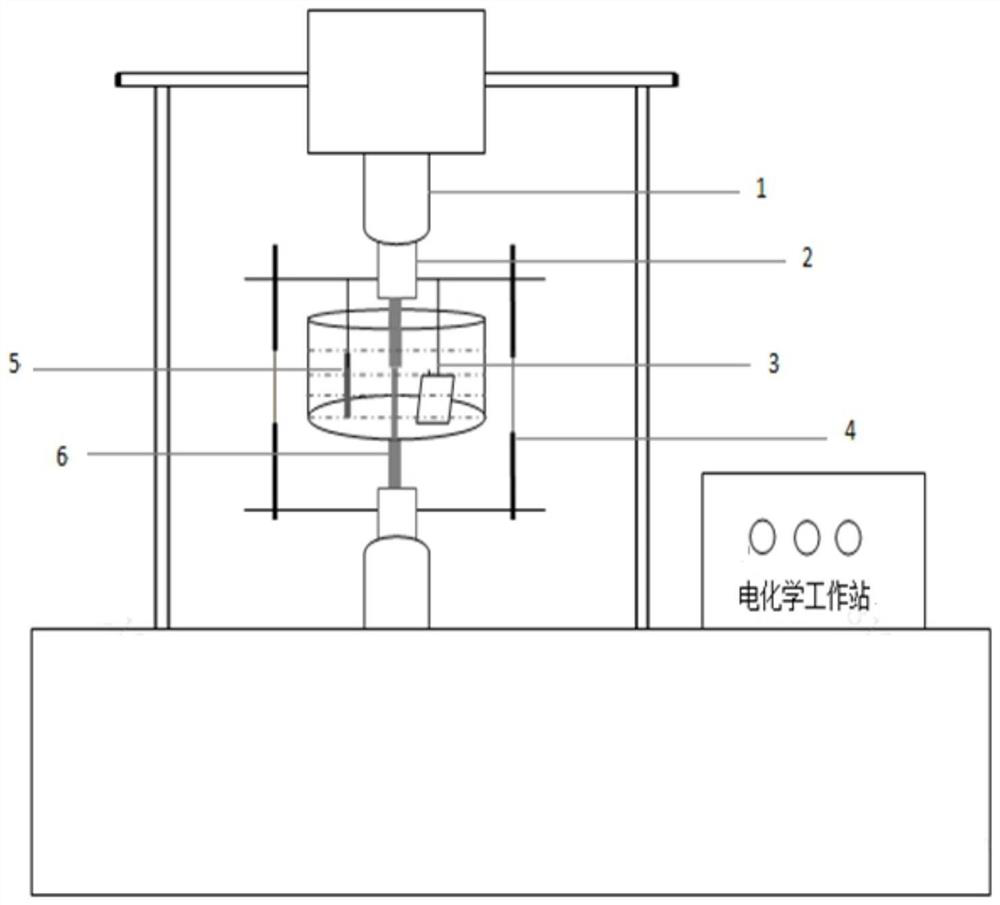
Method of measuring critical current density in hydrogen evolution through reinforced steel bar in reinforced concrete structure
ActiveCN106442682AEasy and quick determinationAccurate measurementMaterial electrochemical variablesWorkstationRebar
Owner:ZHEJIANG UNIV
Buried metal pipeline erosion resistant coating disbonding test system under action of direct current stray current
InactiveCN108507938ASimple structureEasy to operateWeather/light/corrosion resistancePower flowEngineering
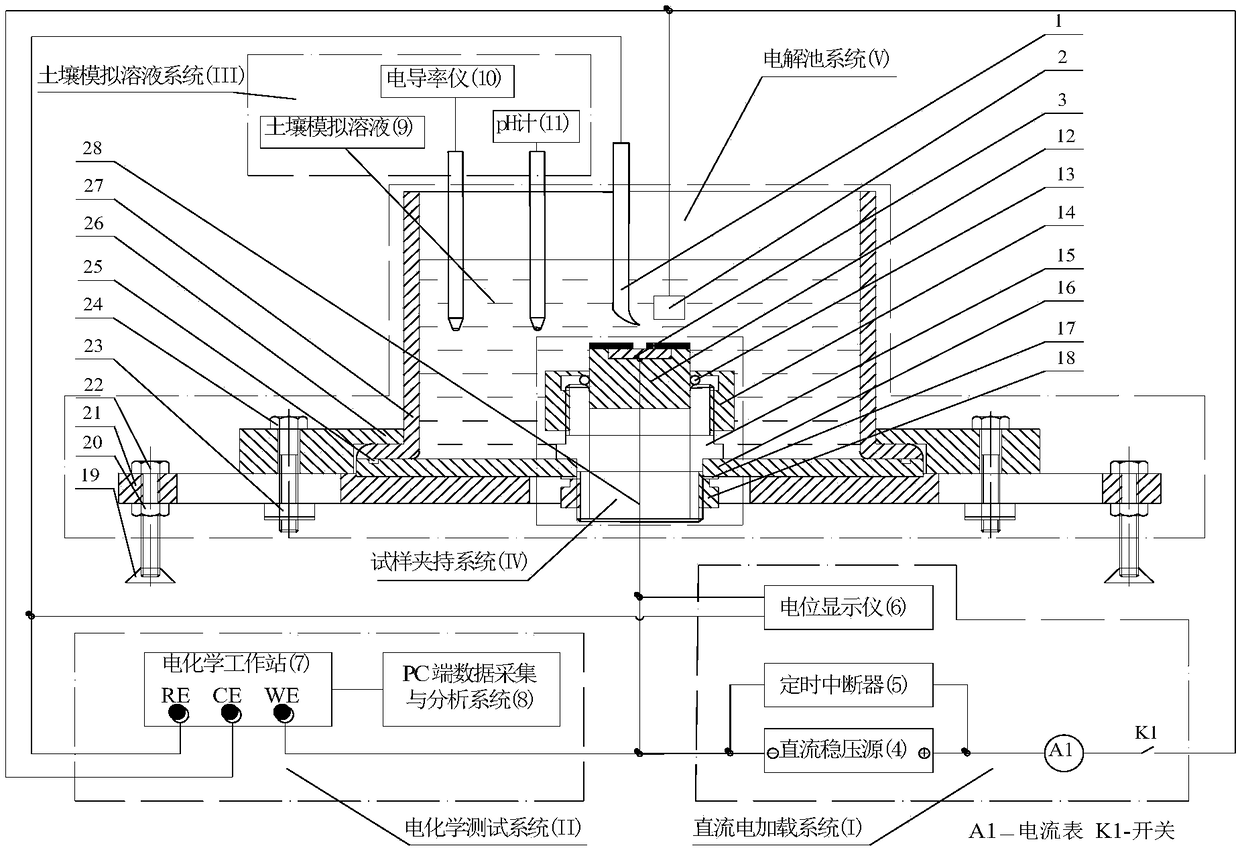
The invention discloses a buried metal pipeline erosion resistant coating disbonding test system under the action of direct current stray current, comprising a direct current stray current system which is used for providing direct current stray current required by sample erosion resistant coating cathodic disbonding; an electrochemical test system which is used for testing the dynamic parameters of sample erosion resistant coating disbonding in the direct current cathodic polarization process and performing data processing and analysis; a soil simulated solution system which is used for simulating the actual working conditions of a buried metal pipeline in different soil environments; a sample clamping system which is used for clamping a sample of the buried metal pipeline needed by test;and an electrolytic tank system which is used for containing the soil simulated solution and fixing the sample clamping system. The system disclosed by the invention is simple in structure, convenientin dismounting, good in experiment test result, capable of effectively simulating pipeline erosion resistant coating cathodic disbonding caused by inflow of the stray current to the damaged points ofthe erosion resistant coating of the buried metal pipeline, and applicable to the experimental study of cathodic disbonding of the erosion resistant coatings of various buried pipelines.
Owner:BEIJING UNIV OF TECH
Surface treatment agent capable of improving corrosion resistance of electrolytic copper foil
The invention belongs to the technical field of electrolytic copper foil surface treatment, and particularly relates to a surface treatment agent capable of improving corrosion resistance of electrolytic copper foil. The surface of the copper foil is electroplated to form an even and exquisite tin-zinc-cerium ternary alloy composite coating, thus corrosion resistance of the copper foil is improved, and the anti-hydrochloric acid cracking rate of the copper foil can be 2% or below. Rare earth element cerium has 4f layer unfilled electronic structure characteristics, small electronegativity, andhigh chemical affinity, can effectively improve performance of plating liquid of a traditional electroplating process, is prone to generating characteristic adsorption on active points of crystal growth, and hinders too fast discharge of metal ions, and thus a structure of the coating is changed; characteristic adsorption of cerium ions is conducive to negative shifting of cathodic deposition potential, the cathodic polarization effect is improved, too fast discharge of tin and zinc ions can be effectively hindered, and thus the even and exquisite alloy coating is obtained; and a preparationmethod is simple, environmentally friendly and low in cost.
Owner:SHANDONG JINBAO ELECTRONICS
Nickel positive electrode for fiber battery
ActiveUS9620770B2Increase productionLarge capacityElectrode manufacturing processesElectrode carriers/collectorsFiberCarbon fibers
Disclosed is a nickel positive electrode for a fiber battery having a long life duration, and also being enabling a high output and high capacity to be attained. For this purpose, the nickel positive electrode for a fiber battery is obtained by coating a carbon fiber with nickel, then causing a cathodic polarization in a nickel nitrate bath using the nickel-coated carbon fiber as a cathode, and then immersing the precipitate, which was deposited on the surface of the carbon fiber by the cathodic polarization, in an aqueous caustic alkali solution.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
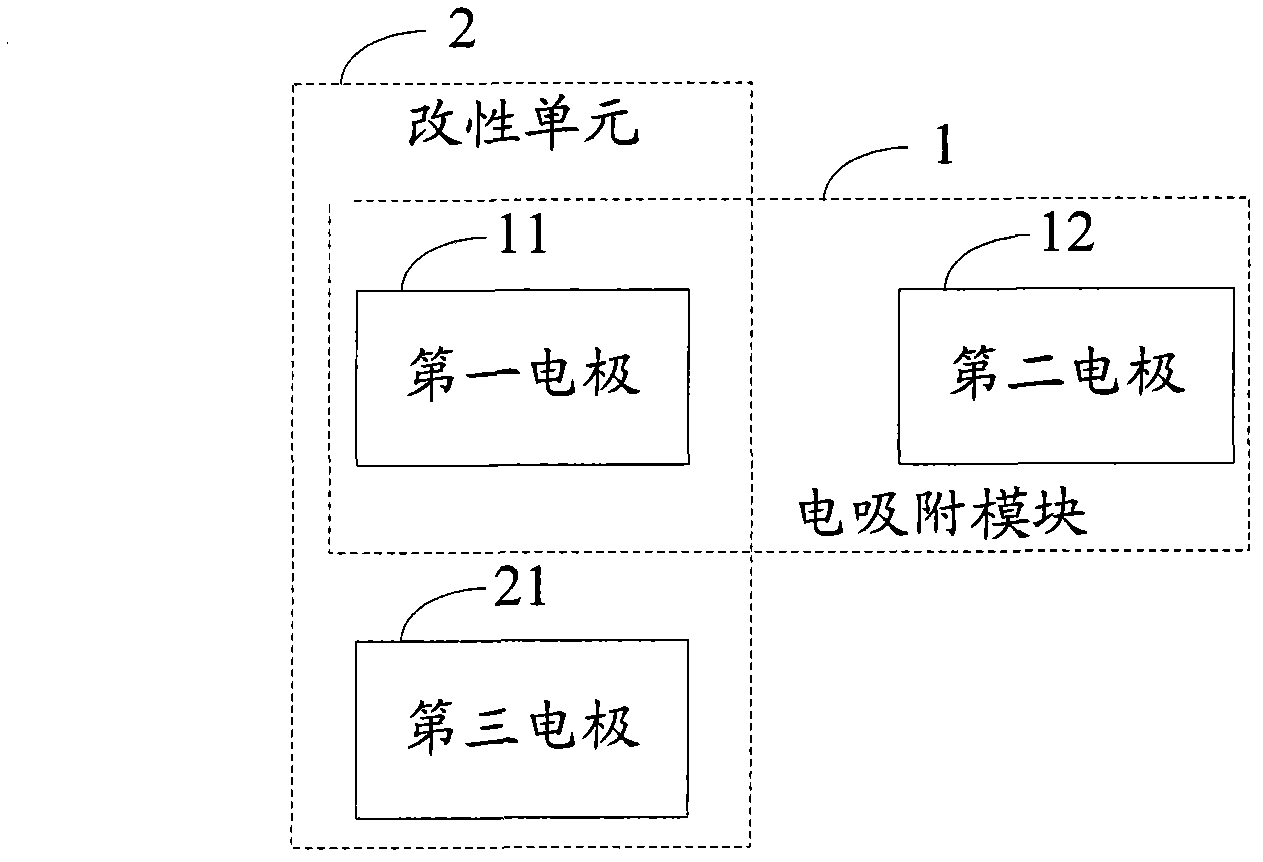
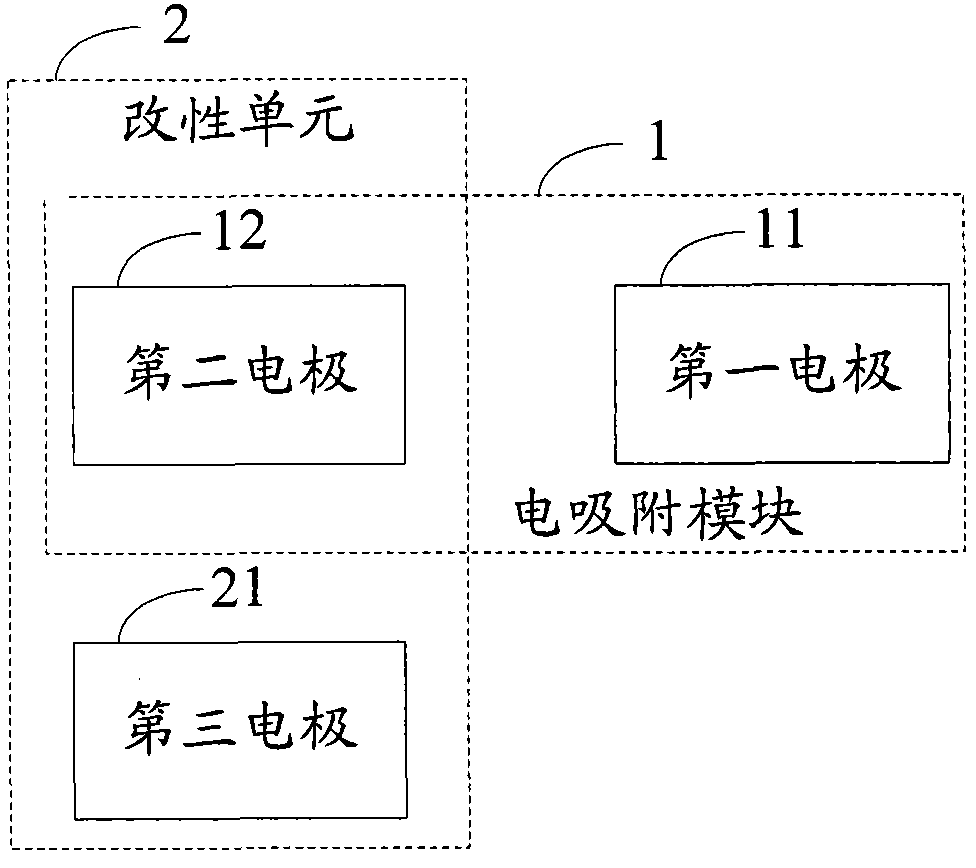
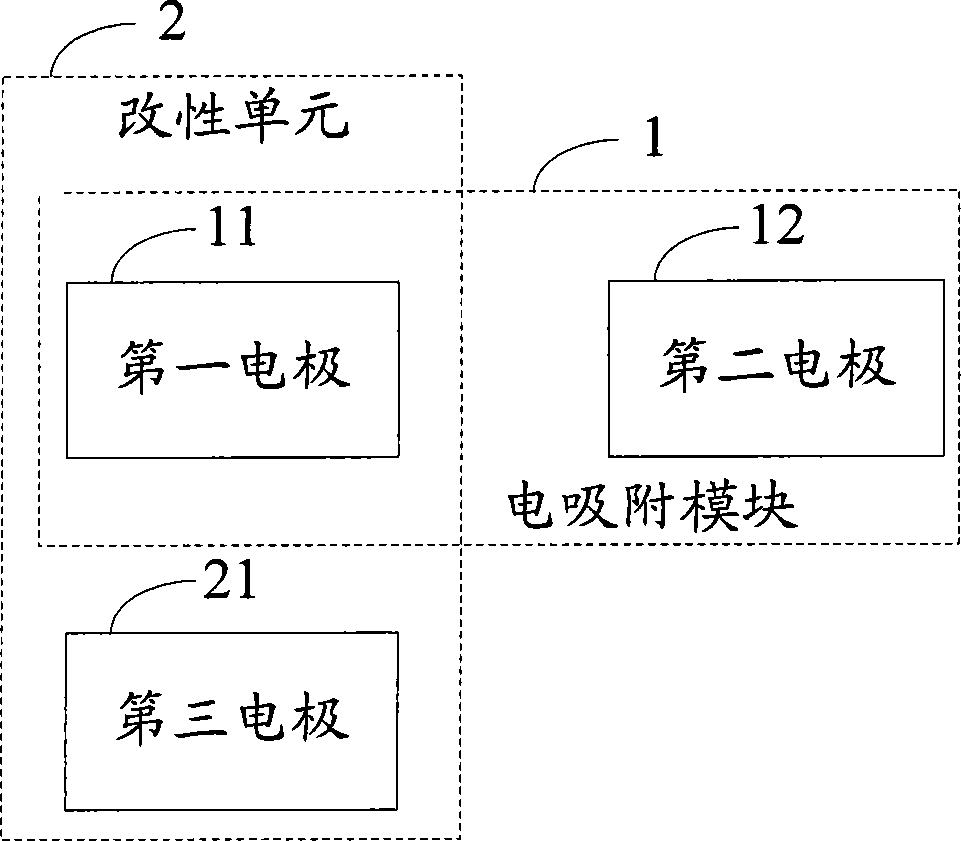
Electro-adsorption module modification system and process
ActiveCN102690004AExtend your lifeDispersed particle separationMultistage water/sewage treatmentElectricityEngineering
The invention discloses an electro-adsorption module modification system and a process, which relate to the field of water treatment, and aim to solve the problem that the desalting efficiency of the electro-adsorption module is affected due to accumulation of surface side reaction effects in a long-time running process of an existing electro-adsorption system. The system comprises a first electrode, a second electrode, a third electrode and a modification unit, wherein the first electrode and the second electrode form the electro-adsorption module when electro-adsorption is carried out; the third electrode is used as a positive electrode when the electro-adsorption module is modified; and the modification unit is formed by the third electrode used as the modified positive electrode, and the first electrode or the second electrode used as the positive electrode during electro-adsorption work while as a negative electrode during modification, and is used for carrying out cathodic polarization on the modified negative electrode so as to complete modification of the electro-adsorption module. The process comprises the following steps of: adopting the third electrode as the modified positive electrode, andthe first electrode or the second electrode as the modified negative electrode; and carrying out cathodic polarization on the first electrode or the second electrode used as the modified negative electrode so as to complete modification of the electro-adsorption module; and the steps are implemented by adopting the system.
Owner:EST WATER & TECH
Cyanide-free copper electroplating method
The invention discloses a cyanide-free copper electroplating method which comprises the following steps of: during the complexation process of copper ions in an electroplate liquid, firstly, complexing the copper ions in the electroplate liquid by using HEDP (Hydroxy Ethidene Phosphonic Acid); then, complexing the residual copper ions in the electroplate liquid by using EDTA (Ethylene Diamine Tetraacetic Acid) in an auxiliary mode. The technical scheme comprises the following steps of: complexing the copper ions in the solution by using a proper complexing agent, and activating a metal matrix; complexing the residual copper ions in the solution by using a proper auxiliary complexing agent, increasing cathodic polarization so that the copper ions are more difficult to discharge to effectively prevent replacement precipitation, and increasing the bonding force of an electroplating layer. The invention has the advantages of obviously increased bonding force of copper electroplated layer, simple technological operation and convenient electroplate liquid maintenance.
Owner:河南平原光电有限公司
Auxiliary brightening agent for potassium chloride galvanizing and preparation method and use of auxiliary brightening agent
The invention provides an auxiliary brightening agent for potassium chloride galvanizing. The auxiliary brightening agent is combined with a main brightening agent and a carrier brightening agent for application, and is characterized in that the formula of the auxiliary brightening agent comprises natural gum, wherein the natural gum is crushed into powder, the powder is dissolved in hot water, impurities are filtered from the powder through a filter, and the powder is added into the auxiliary brightening agent for uniform stirring. According to the auxiliary brightening agent, the cathodic polarization of a high-current density area can be improved, the dispersion capability of a plating solution can be improved, and plated layer crystals are delicate and bright; the plating solution is high in covering power, and a plated layer is clear, smooth and high in deposition speed, the binding force of the plated layer can meet requirements, high current efficiency is ensured, and a plated part is prevented from being scorched and blackened; the auxiliary brightening agent is easy to prepare, stable, low in cost, pollution-free and environmentally friendly.
Owner:王维福
Method for measuring most negative cathodic protection potential of steel
PendingCN114720255AEasy to corrodeInhibition of oxygen absorption corrosionWeather/light/corrosion resistanceMaterial strength using tensile/compressive forcesAnalysis dataPhysical chemistry
Owner:JIANGYIN XINGCHENG SPECIAL STEEL WORKS CO LTD
Cathode powder of cylindrical basic zinc-manganese battery and preparation method of cathode powder
The invention provides cathode powder of a cylindrical basic zinc-manganese battery and a preparation method of the cathode powder. The cathode powder comprises the following component in part by weight: 80 to 90 parts of electrolytic manganese dioxide, 1 to 10 parts of graphite powder, 0.5 to 1 part of calcium stearate and 1 to 5 parts of ethanol. The cathode powder and the preparation method are low in raw material cost and have a simple preparation process; and by the preparation method, the surface state of cathode powder particles can be improved, porous particles are formed, and the conditions that the reaction area of a cathode is increased, discharge density is reduced, anodic polarization is relieved and the discharge performance of large current is improved are facilitated, so that the battery is particularly suitable for large-current electrical appliances such as a digital camera and the like.
Owner:浙江特源电池有限公司 +1
Method for improving mechanical property of electrolytic copper foil and additive used by method
ActiveCN113430586ALow costReduce pollutionPolycrystalline material growthFrom normal temperature solutionsElectrolysisCopper foil
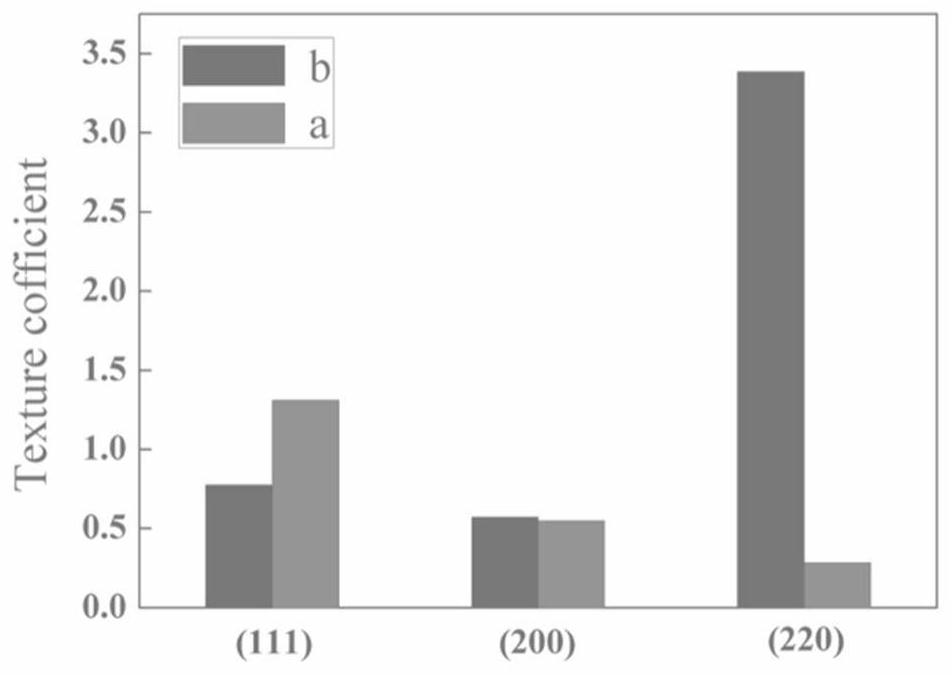
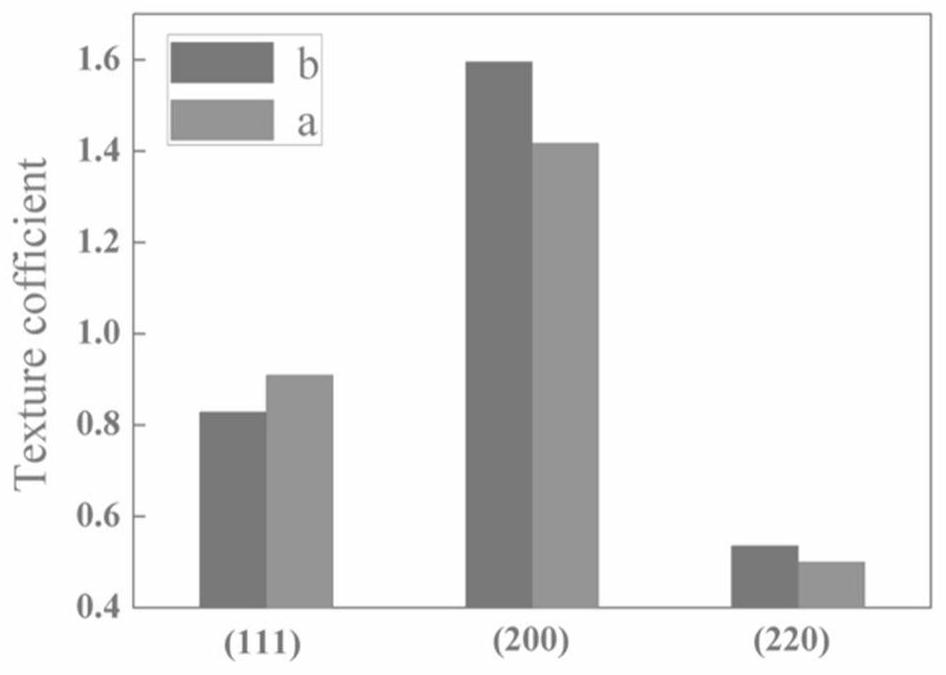
The invention relates to a method for improving the mechanical property of an electrolytic copper foil and an additive used by the method, and belongs to the field of lithium ion batteries. The method solves the problems that the mechanical property of the existing electrolytic copper foil is weak, the tensile property is reduced along with the reduction of the thickness, and the problems of high cost, high process difficulty and the like generally exist in a process for specifically strengthening the mechanical property of the electrolytic copper foil. According to the method for improving the mechanical property of the electrolytic copper foil, in the preparation process of the electrolytic copper foil, the crystal face growth trend of crystal grains in the copper foil is controlled, the (220) crystal face growth of copper crystal grains is promoted, the texture coefficient of copper grains on the (220) crystal face is increased, and / or cathodic polarization in the copper deposition process is increased, and the copper grains are refined. The method can effectively reduce cost and pollution; the modification treatment mode is simple and efficient, and extra procedures are omitted; the growth orientation of copper grains can be induced, and the orientation of the crystal face (220) is enhanced; and meanwhile, grain refinement can be realized, and the mechanical property of the copper foil is strengthened.
Owner:ZHEJIANG UNIV OF TECH
Electrochemical coupling of metallic biomaterial implants for biological effect
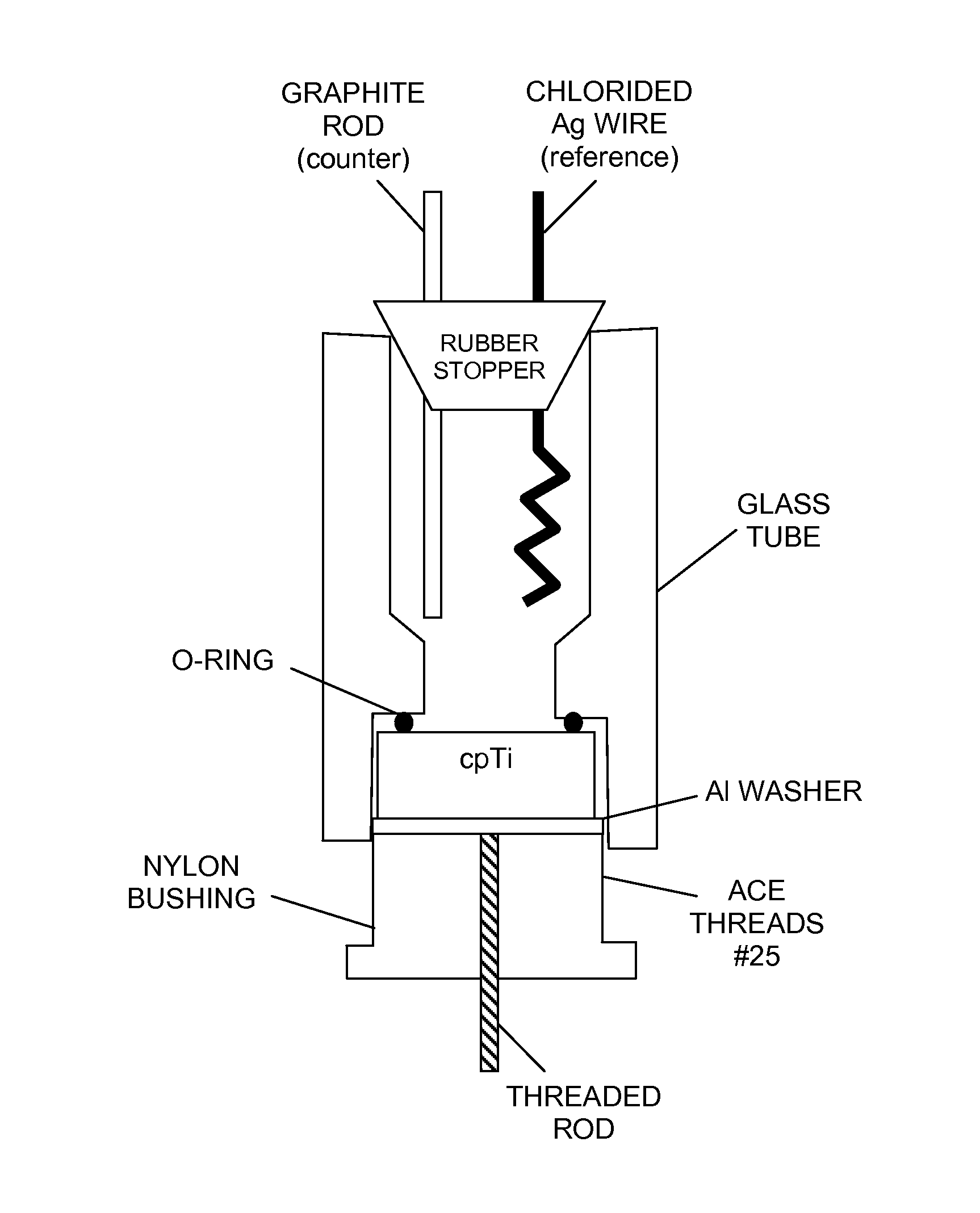
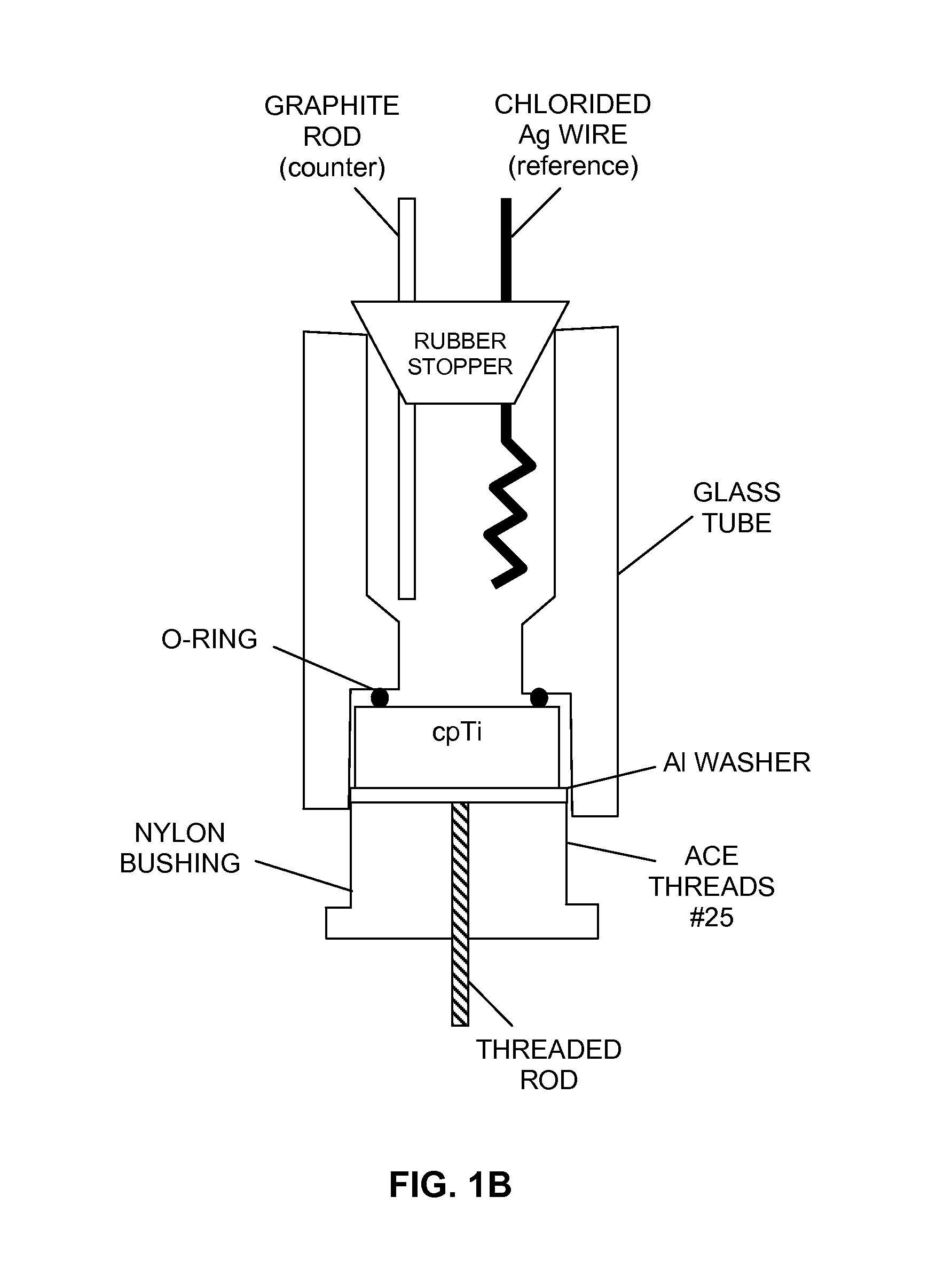
ActiveUS9039764B2Reduce inflammationPrevent bacterial growthDental implantsBone implantCouplingTitanium
The invention discloses a novel method of controlling the open circuit potential (OCP) of a medical implant by coupling it with small amounts of metals having a lower OCP than the implant. Coupling of Mg to less than 1% of the surface area of a titanium implant is shown to induce cathodic polarization of the titanium that inhibits cell proliferation at the surface of the implant. Mg—Ti coupling in medical devices promises to attenuate or eliminate potential complications of surgery such as peri-implantitis and bacterial infections at the site of implantation.
Owner:SYRACUSE UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com