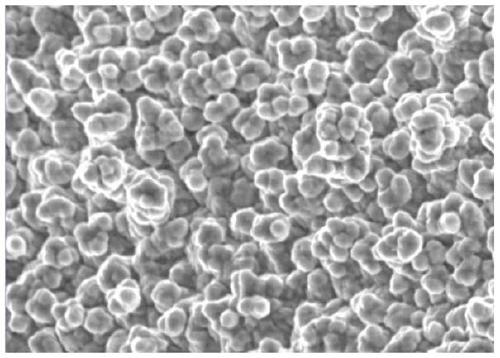
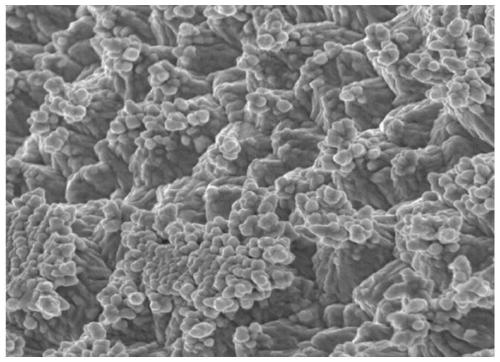
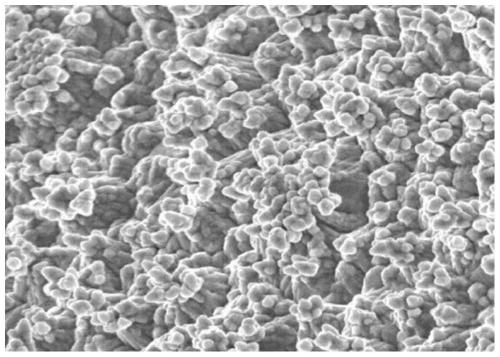
Surface treatment agent capable of improving corrosion resistance of electrolytic copper foil
A surface treatment agent and corrosion-resistant technology, applied in the field of electrolytic copper foil surface treatment, can solve the problems of insufficient crystallisation of the alloy layer, edge corrosion, high cost, increase the cathodic polarization, improve corrosion resistance, The effect of strong chemical affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A surface treatment agent for improving the corrosion resistance of electrolytic copper foil. Each liter of the surface treatment agent contains: 130g of potassium pyrophosphate, 3g of stannous sulfate, 18g of zinc sulfate, 1g of cerium sulfate and 3mg of additive A;
[0019] Wherein, Additive A is formaldehyde and 1,4-butynediol mixed in a weight ratio of 1:1.
[0020] A surface treatment process for improving the corrosion resistance of electrolytic copper foil. Using the above-mentioned surface treatment agent, the roughened and cured electrolytic copper foil with a thickness of 18 μm is placed in an electrolyte tank containing the surface treatment agent, and the pH is controlled to 9.0, the temperature is 35°C, the current density is 1.5A / dm 2 , The electroplating time is 8s.
Embodiment 2
[0022] A surface treatment agent for improving the corrosion resistance of electrolytic copper foil. Each liter of the surface treatment agent contains: 170g of potassium pyrophosphate, 8g of stannous sulfate, 27g of zinc sulfate, 5g of cerium sulfate and 9mg of additive A;
[0023] Among them, Additive A is SPS and 1,4-butynediol mixed according to the weight ratio of 1:2.
[0024] A surface treatment process for improving the corrosion resistance of electrolytic copper foil. Using the above-mentioned surface treatment agent, the roughened and cured electrolytic copper foil with a thickness of 18 μm is placed in an electrolyte tank containing the surface treatment agent, and the pH is controlled to 10.5, the temperature is 45℃, the current density is 3A / dm 2 , The electroplating time is 6s.
Embodiment 3
[0026] A surface treatment agent for improving the corrosion resistance of electrolytic copper foil. Each liter of the surface treatment agent contains: 135g of potassium pyrophosphate, 4g of stannous sulfate, 20g of zinc sulfate, 2g of cerium sulfate and 4mg of additive A;
[0027] Among them, additive A is SPS, formaldehyde and 1,4-butynediol mixed according to the weight ratio of 1:2:2.
[0028] A surface treatment process for improving the corrosion resistance of electrolytic copper foil. Using the above-mentioned surface treatment agent, the roughened and cured electrolytic copper foil with a thickness of 18 μm is placed in an electrolyte tank containing the surface treatment agent, and the pH is controlled to 9.5, the temperature is 40℃, the current density is 2.3A / dm 2 , The electroplating time is 6.5s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com