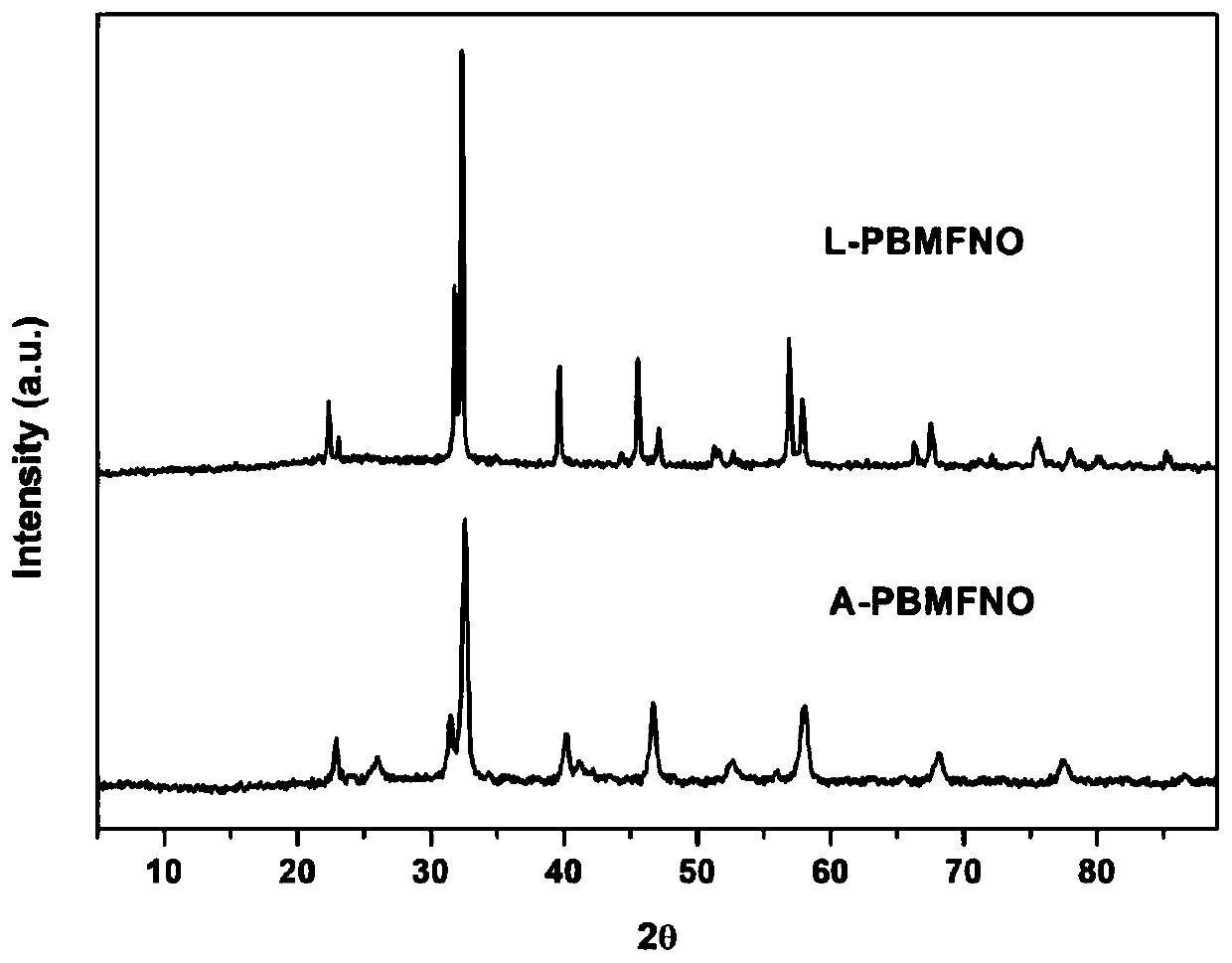
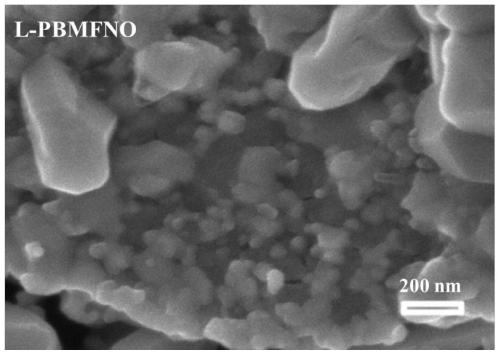
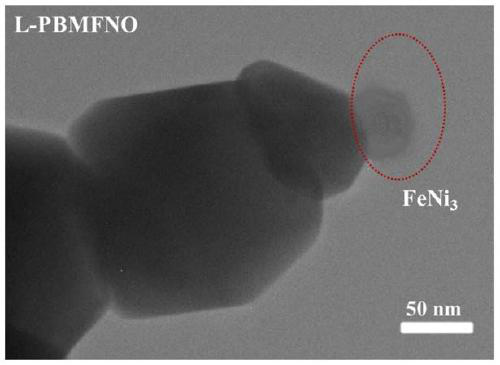
Iron-nickel alloy in-situ desolventized layered perovskite cathode material for CO2 electrolysis
A technology of cathode materials and electrolyte materials, applied in the field of cathode materials for solid oxide electrolytic cells, can solve problems such as insufficient electrocatalytic activity, achieve the effects of improving electrocatalytic reduction activity, combining tightly, and increasing the concentration of oxygen vacancies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: According to Pr 0.45 Ba 0.45 Mn 0.5 Fe 0.4 Ni 0.1 O 3-δ The stoichiometric ratio of Pr, Ba, Mn, Fe and Ni in (A-PBMFNO) calculates the nitrate mass required by different elements. First dissolve these nitrates in deionized water, stir thoroughly for about half an hour, and then add 59.8899 g of citric acid monohydrate and 300 mL of ethylene glycol. At the same time, quickly adjust the pH of the solution to between 8-9 with ammonia water, and then continue to stir until a blood-red, clear and transparent precursor sol is formed. The speed of the magnetic stirrer is 400-500r / min, and the stirring time is 12h.
[0032] Step 2: The prepared precursor sol is transferred to the crucible, and a low-temperature self-propagating reaction is induced by heating by resistance wire. The temperature of the heater is controlled at 350°C, and the organic components, water, ammonia and other components in the precursor are gradually evaporated until a red-brown gel is formed. Aft...
Embodiment 2
[0038] Step 1: According to Pr 0.45 Ba 0.45 Mn 0.5 Fe 0.48 Ni 0.02 O 3-δ The stoichiometric ratio of Pr, Ba, Mn, Fe and Ni elements in the stoichiometric ratio respectively calculates the required nitrate mass of different elements. First dissolve these nitrates in deionized water, stir thoroughly for about half an hour, and then add 59.8899 g of citric acid monohydrate and 300 mL of ethylene glycol. At the same time, quickly adjust the pH of the solution to between 8-9 with ammonia water, and then continue to stir until a blood-red, clear and transparent precursor sol is formed. The speed of the magnetic stirrer is 400-500r / min, and the stirring time is 12h.
[0039] Step 2: The prepared precursor sol is transferred to the crucible, and a low-temperature self-propagating reaction is induced by heating by resistance wire. The temperature of the heater is controlled at 350°C, and the organic components, water, ammonia and other components in the precursor are gradually evaporated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com