Patents
Literature
475 results about "Water-gas shift reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
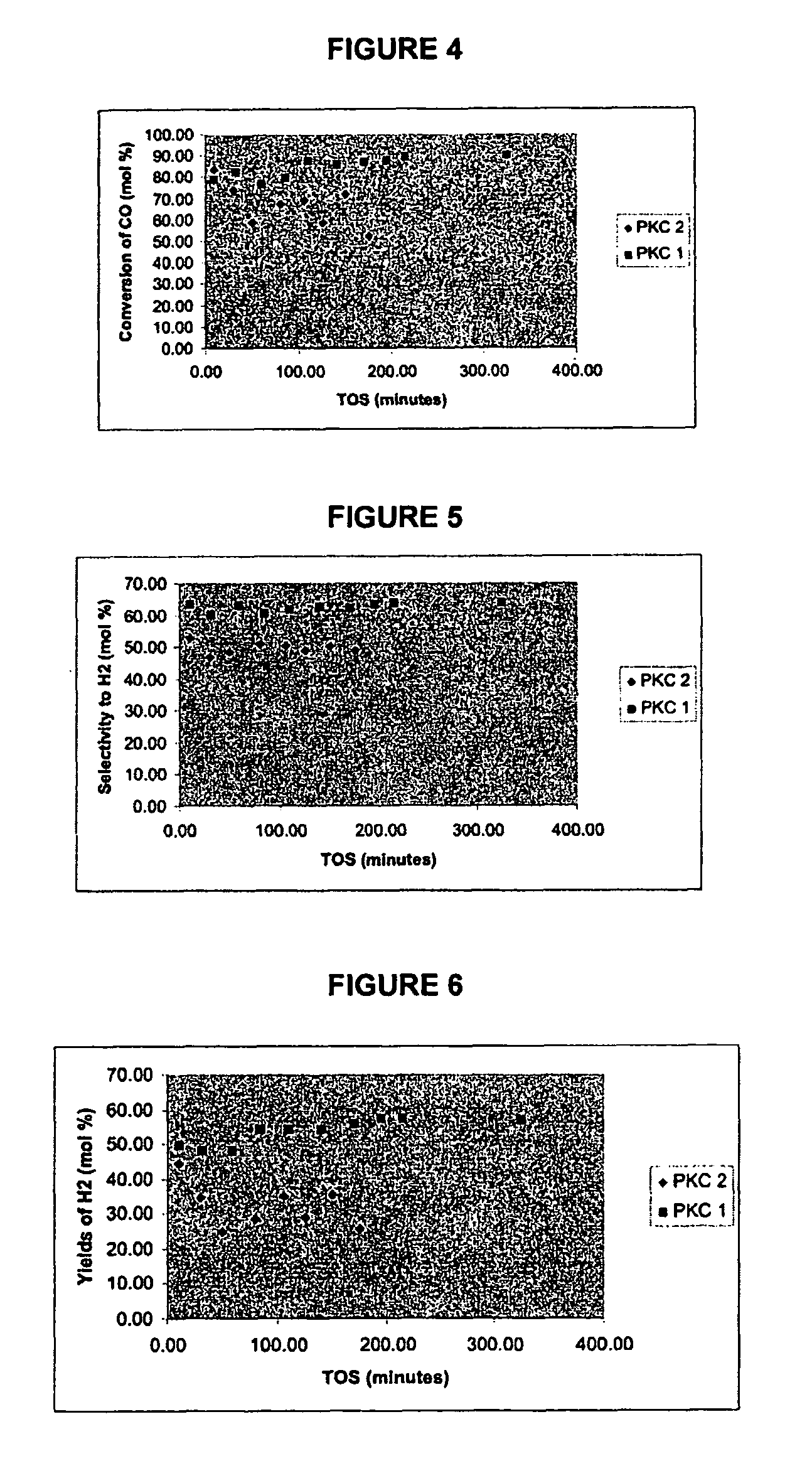
The water-gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen {the mixture of carbon monoxide and hydrogen (not water) is known as water gas}...
Reforming system for combined cycle plant with partial CO2 capture
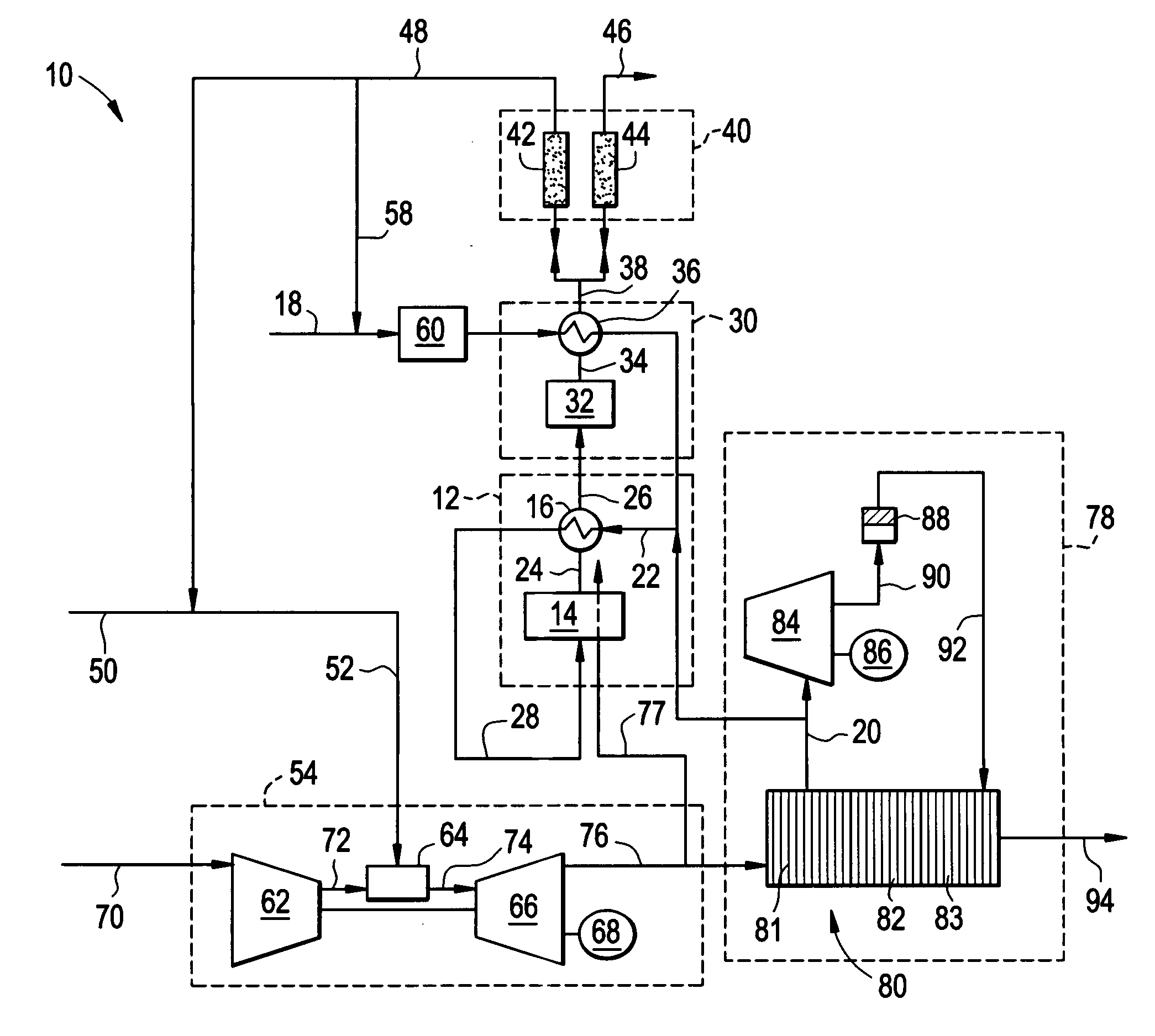
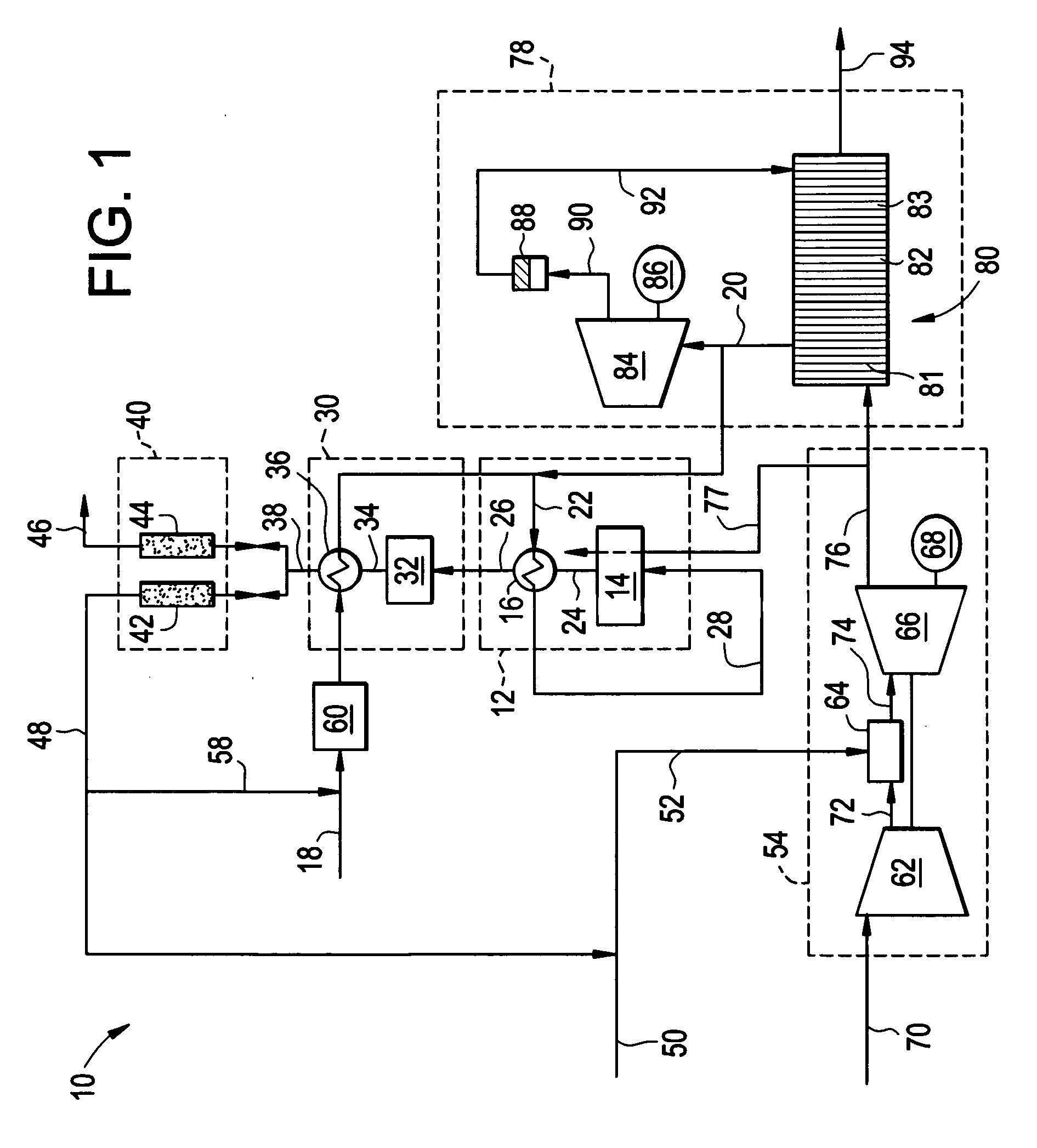
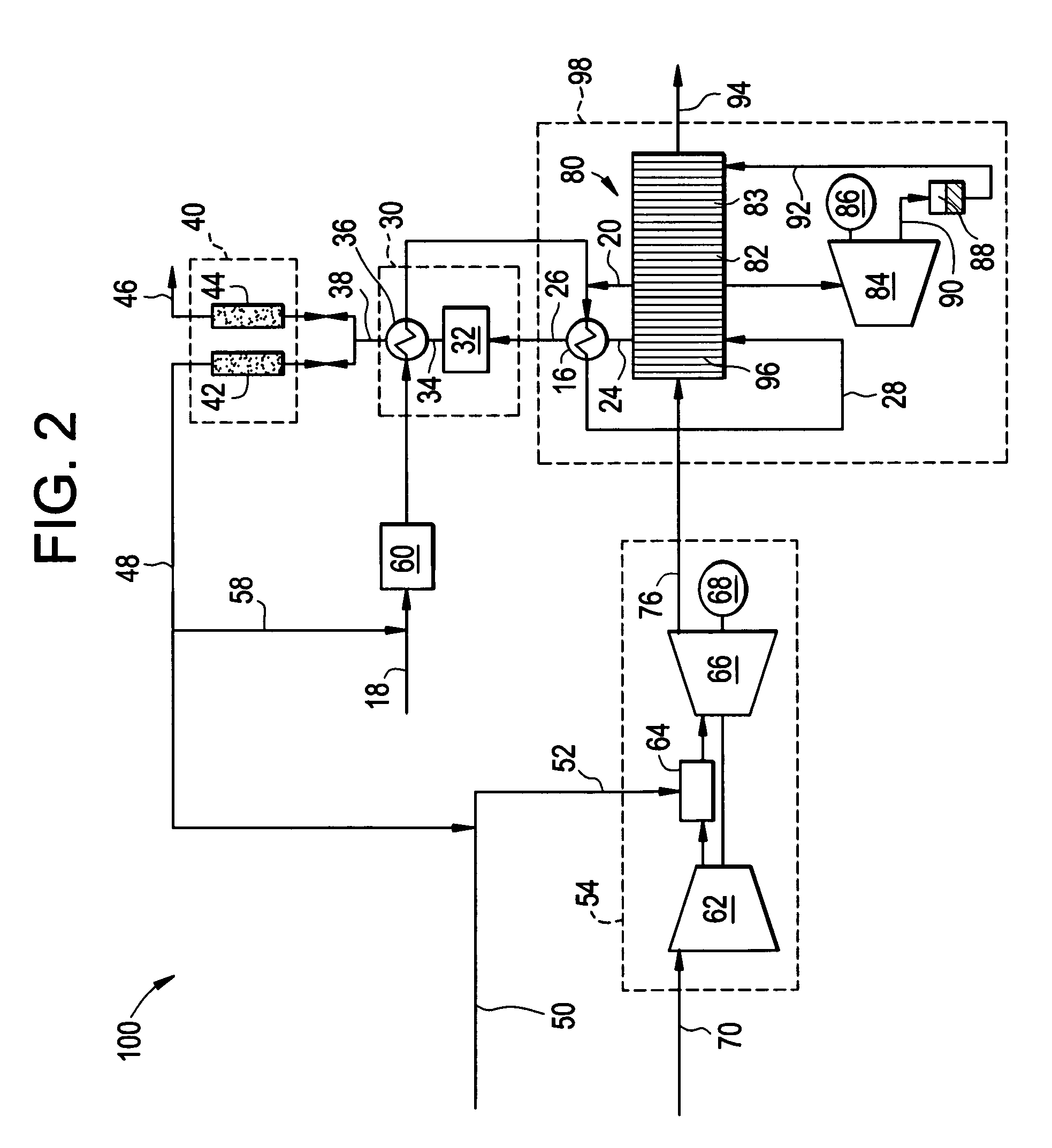
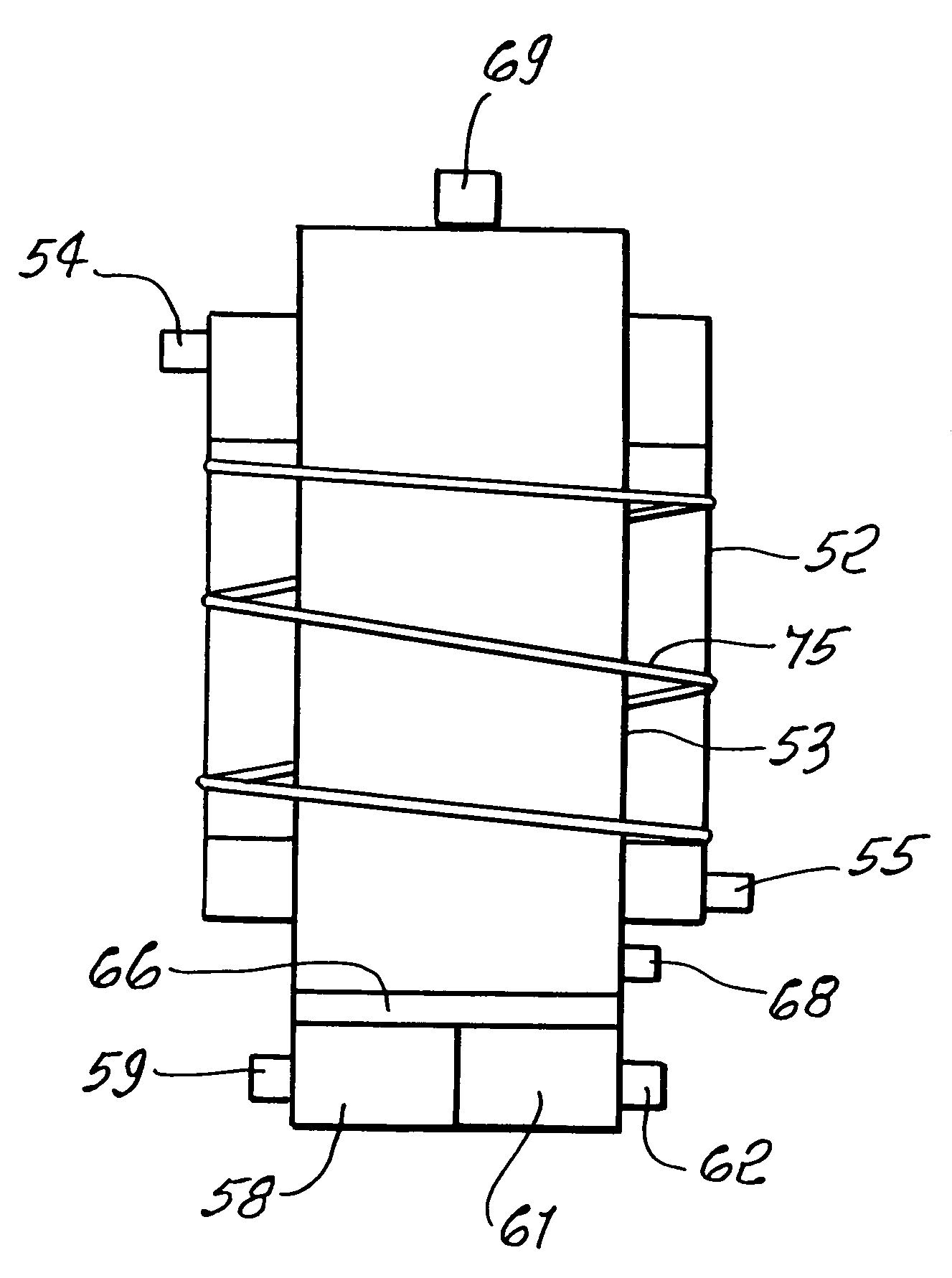
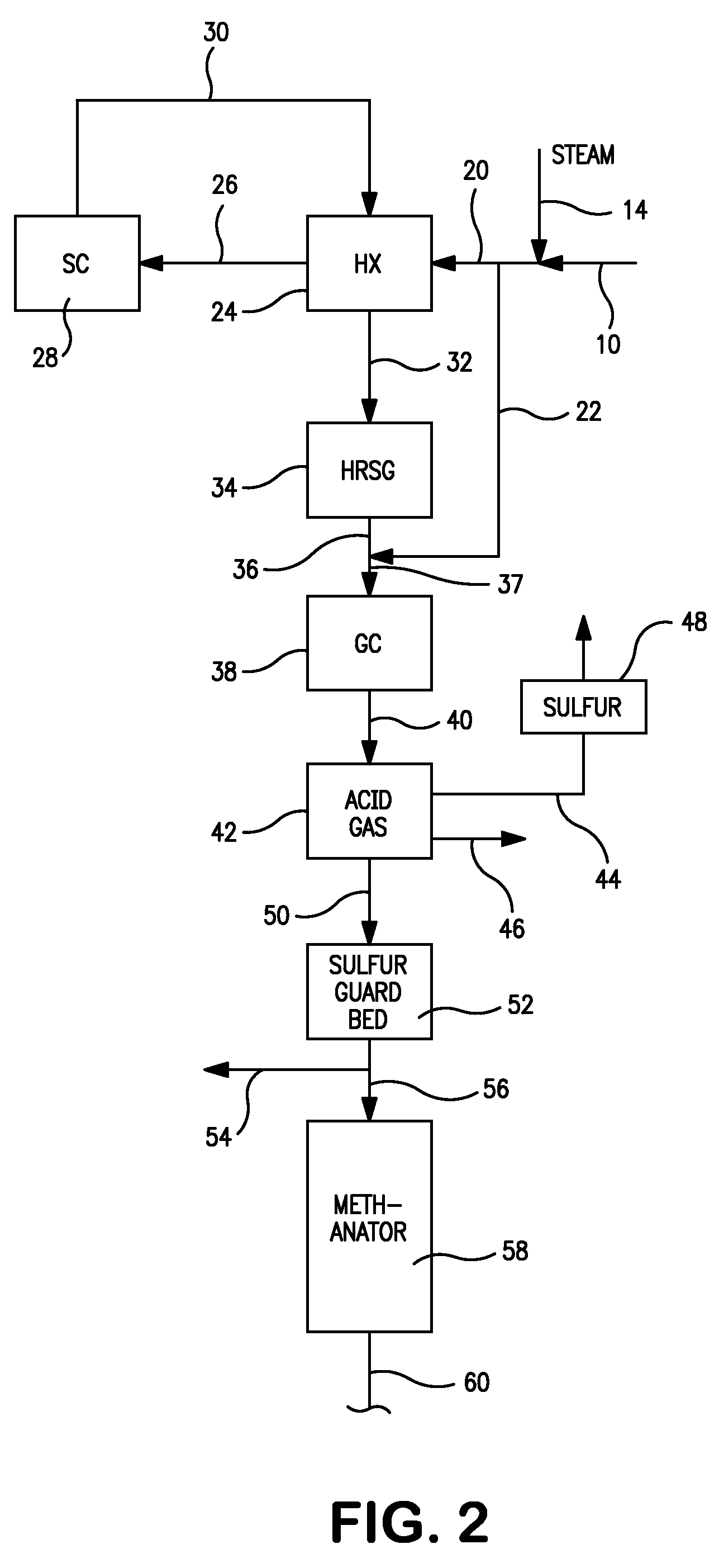
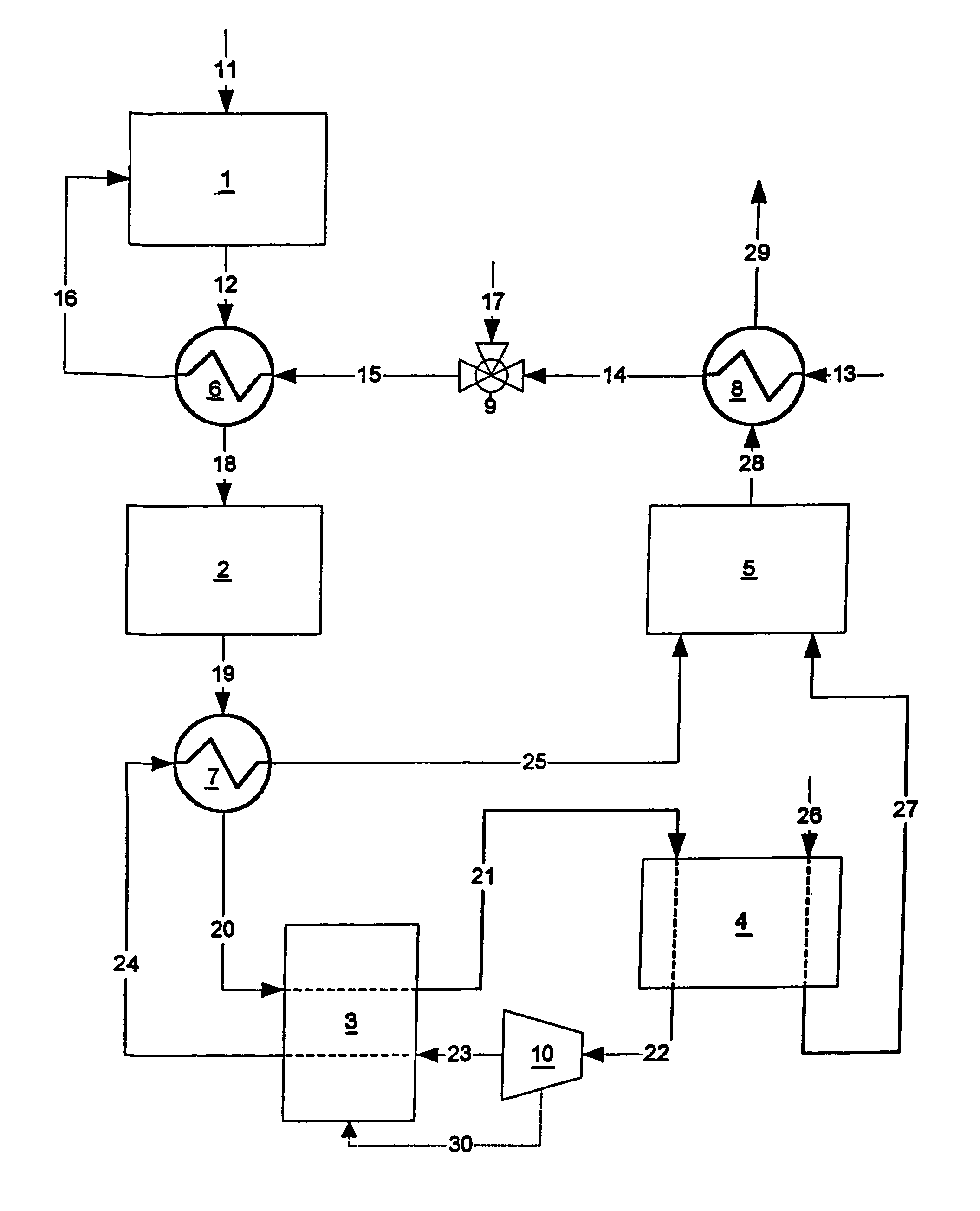
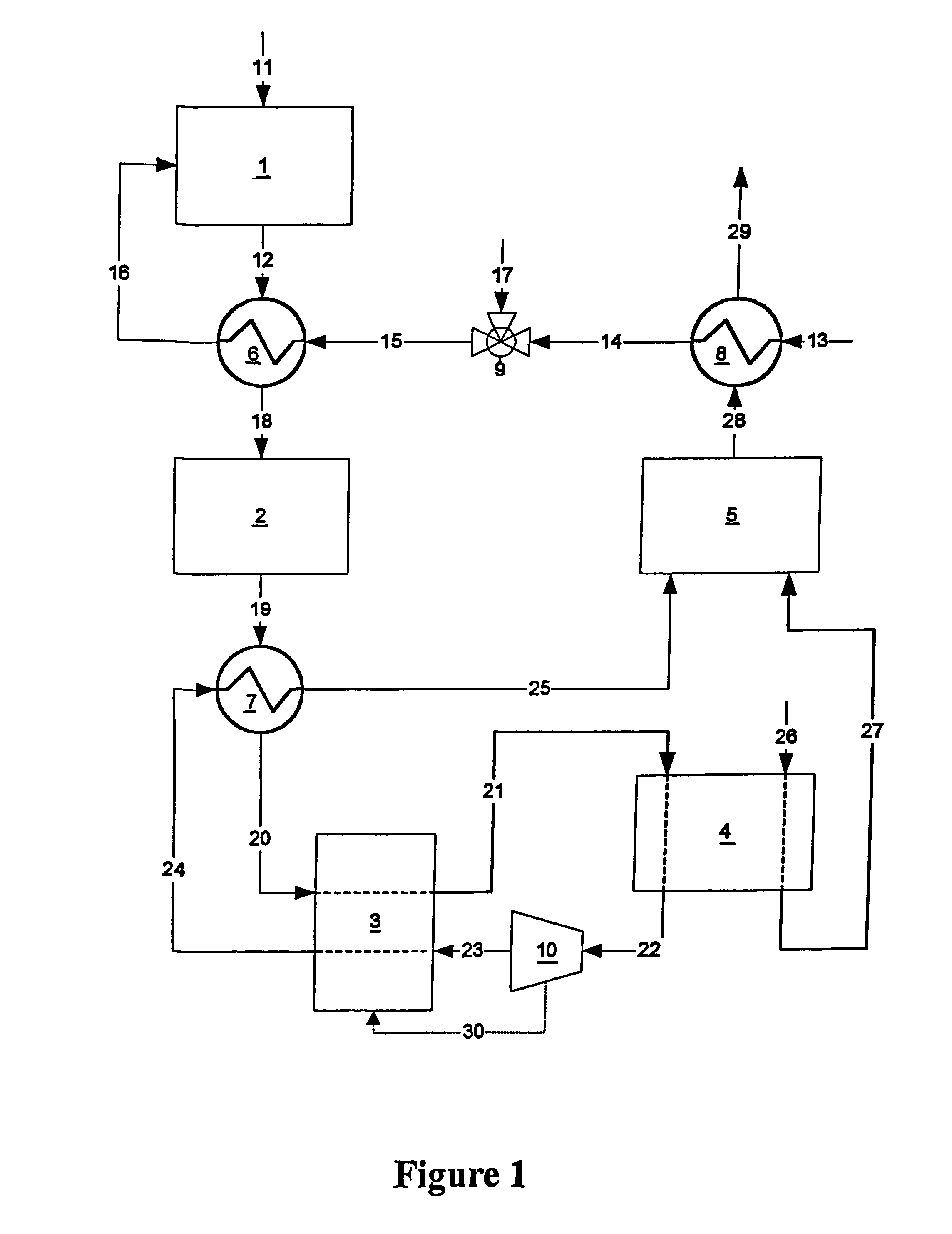
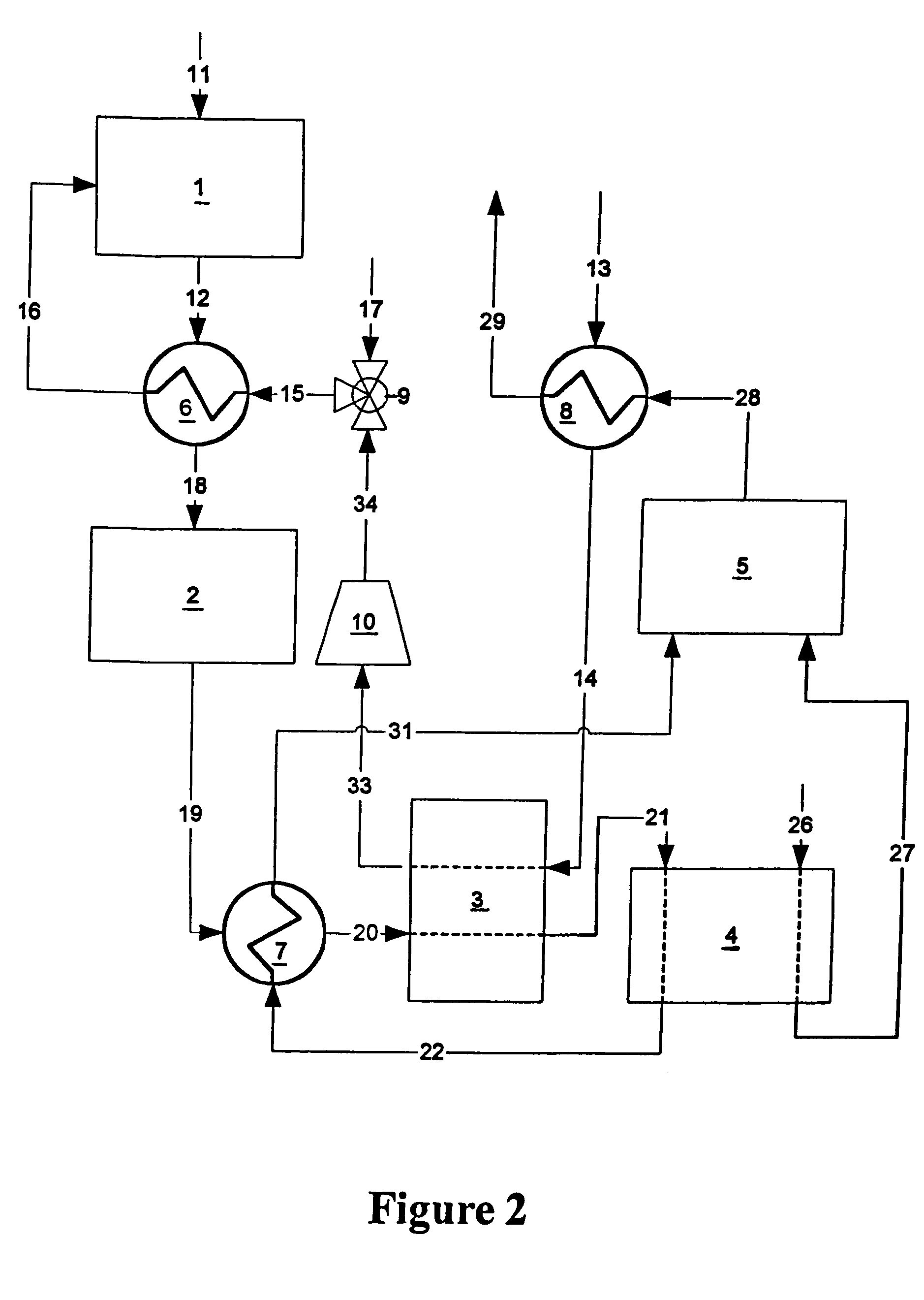
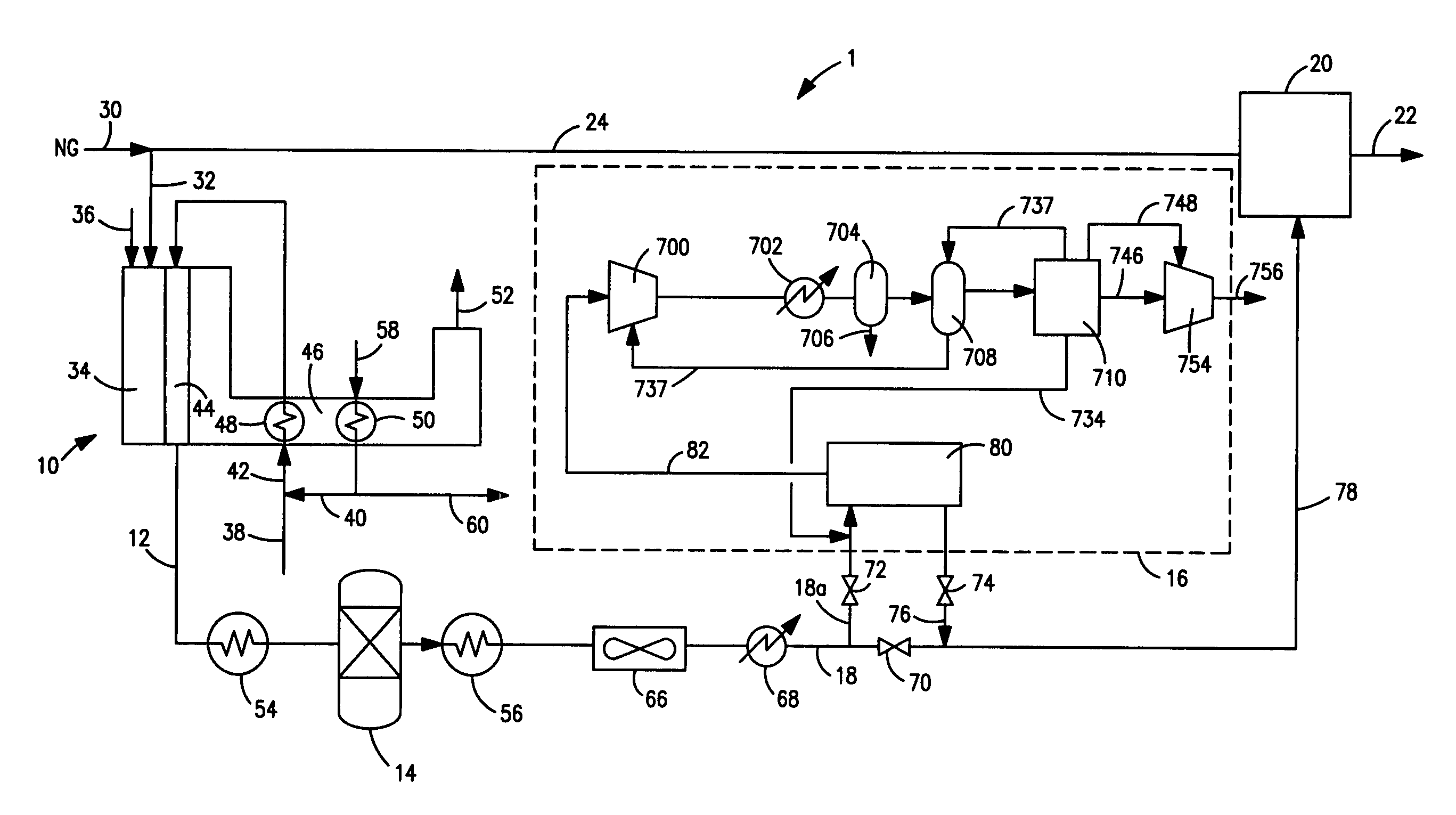
A combined cycle system includes, a pre-steam-methane-reformer operating at a temperature of less than about 800 degrees Celsius to reform a mixed fuel stream to generate a first reformate stream, a water-gas-shift reactor to convert carbon monoxide in the first reformate stream to carbon dioxide and form a second reformate stream, a carbon dioxide removal unit for removing carbon dioxide from the second reformate stream and form a carbon dioxide stream and a third reformate stream; wherein less than about 50 percent of the carbon contained in the mixed fuel stream is recovered as carbon dioxide by the removal unit, a gas turbine unit for generating power and an exhaust stream, and a steam generator unit configured to receive the exhaust stream, wherein the heat of the exhaust stream is transferred to a water stream to generate the steam for the mixed fuel stream and for a steam turbine.
Owner:GENERAL ELECTRIC CO
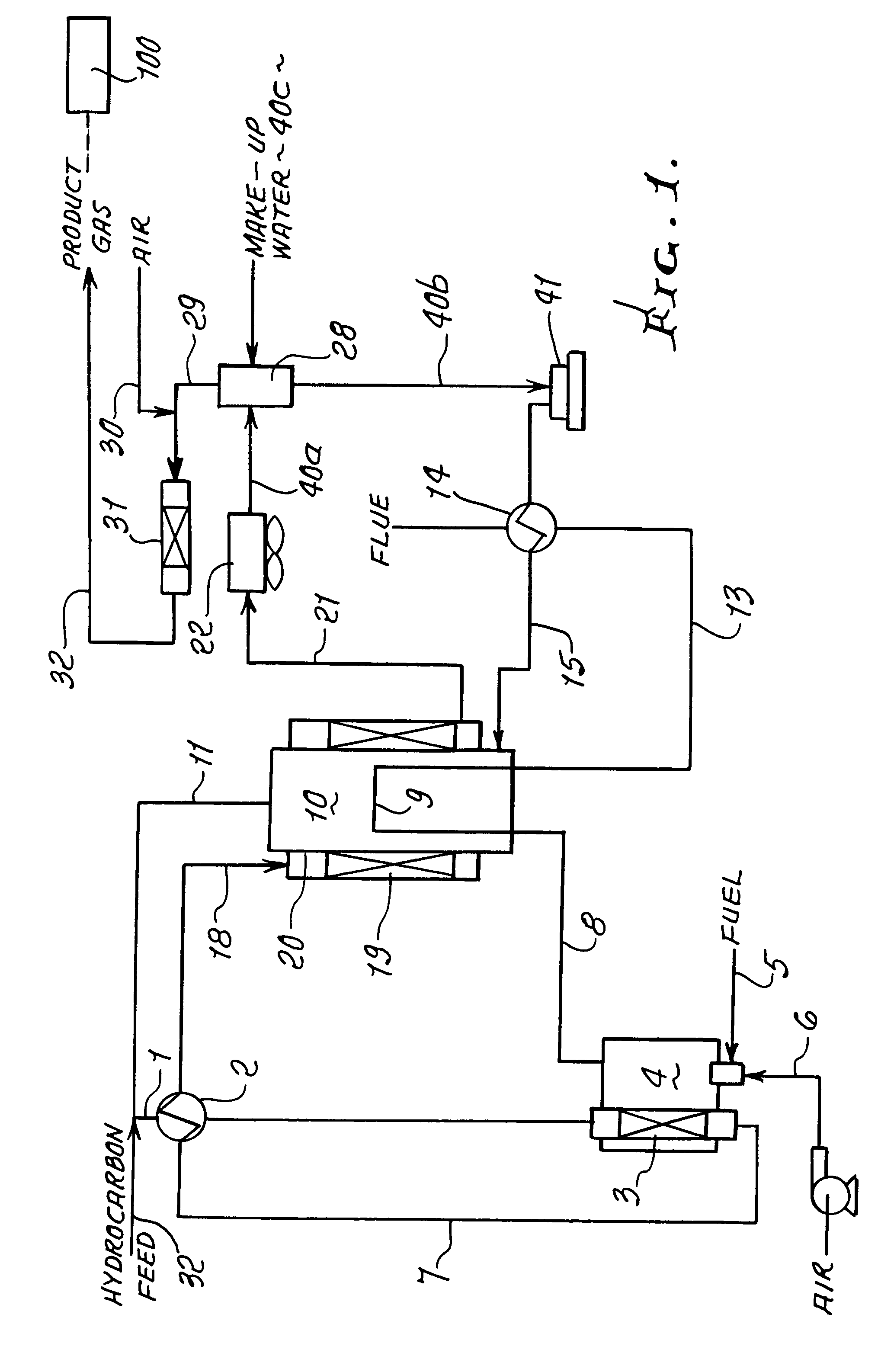
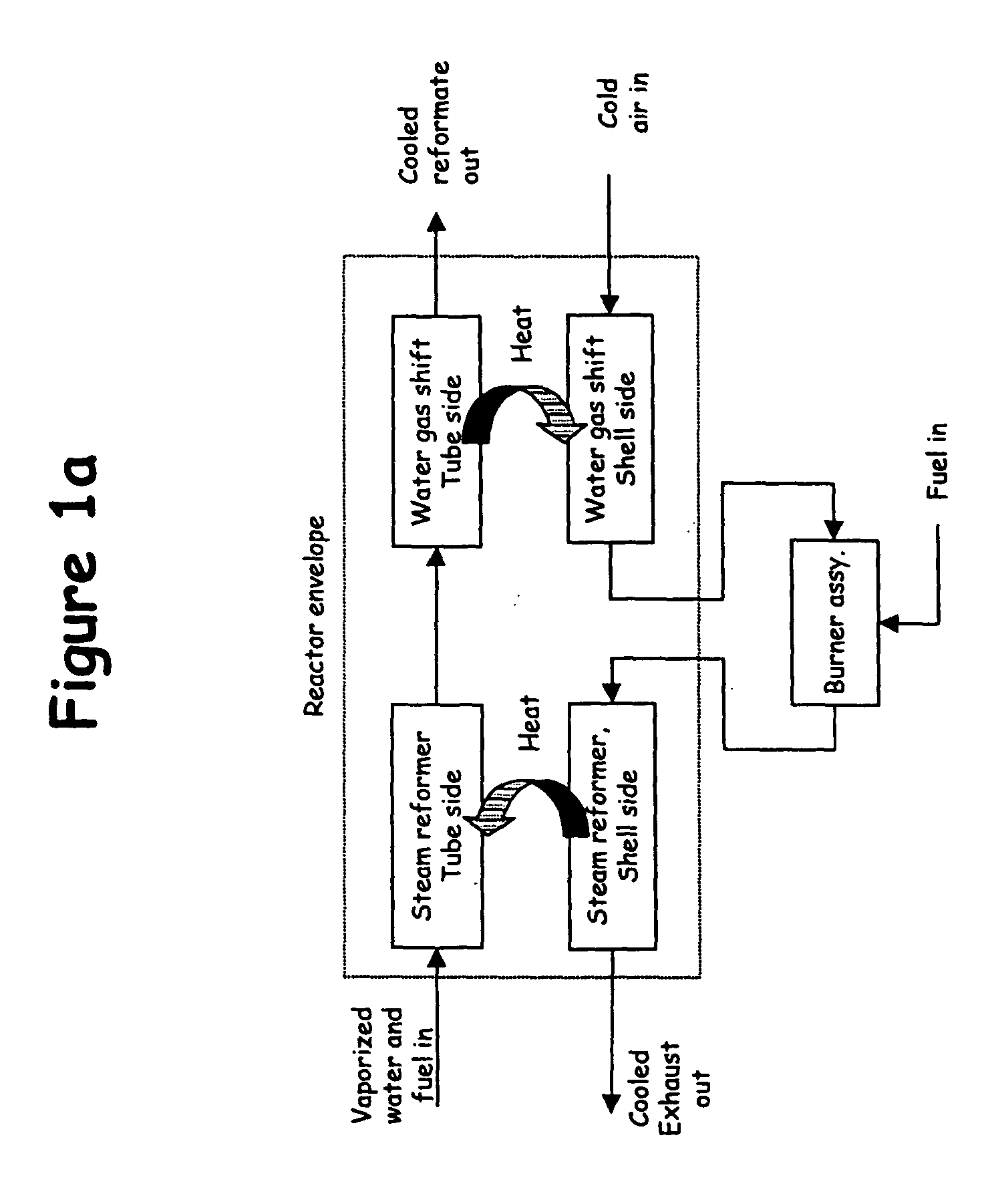
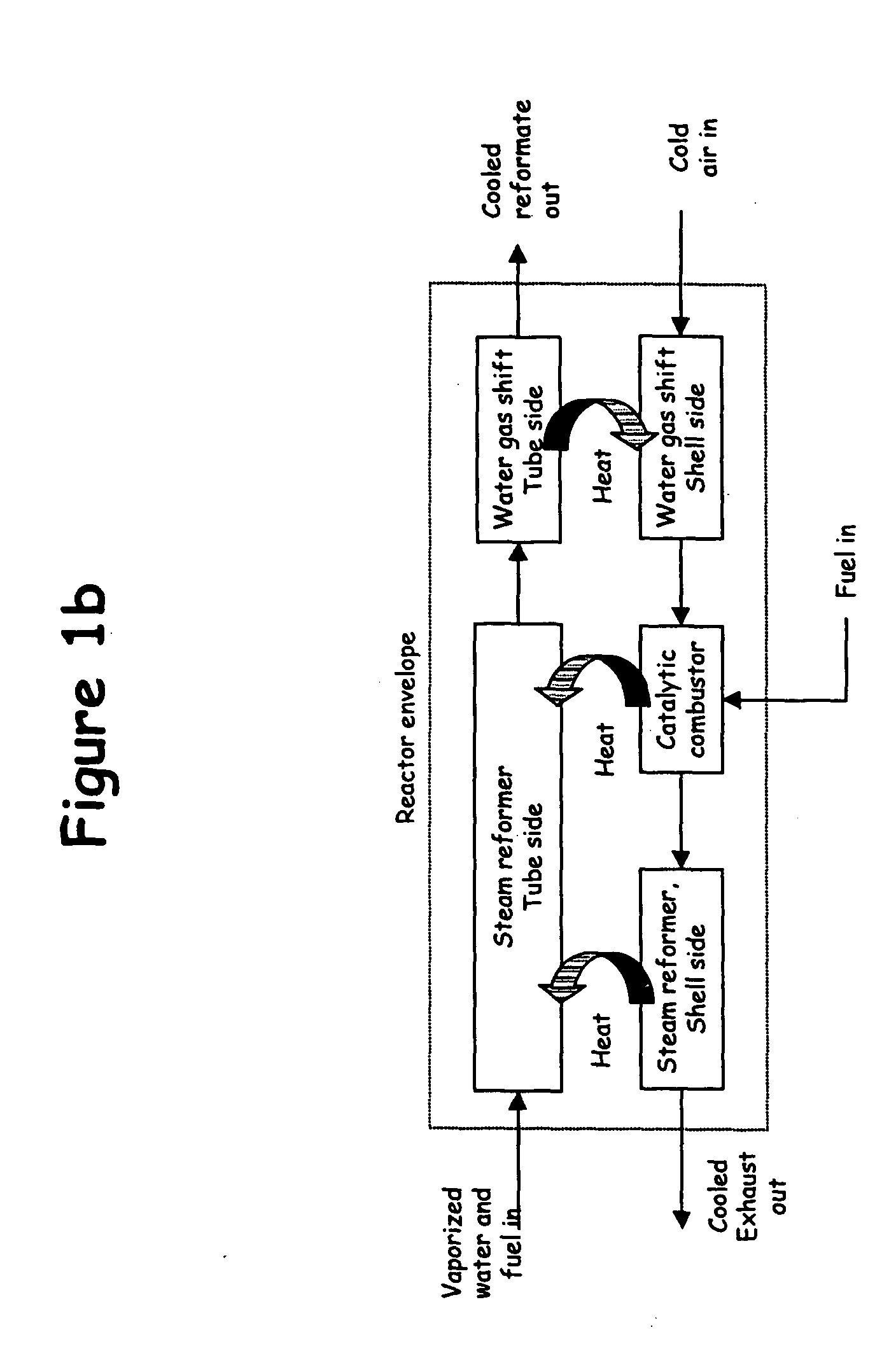
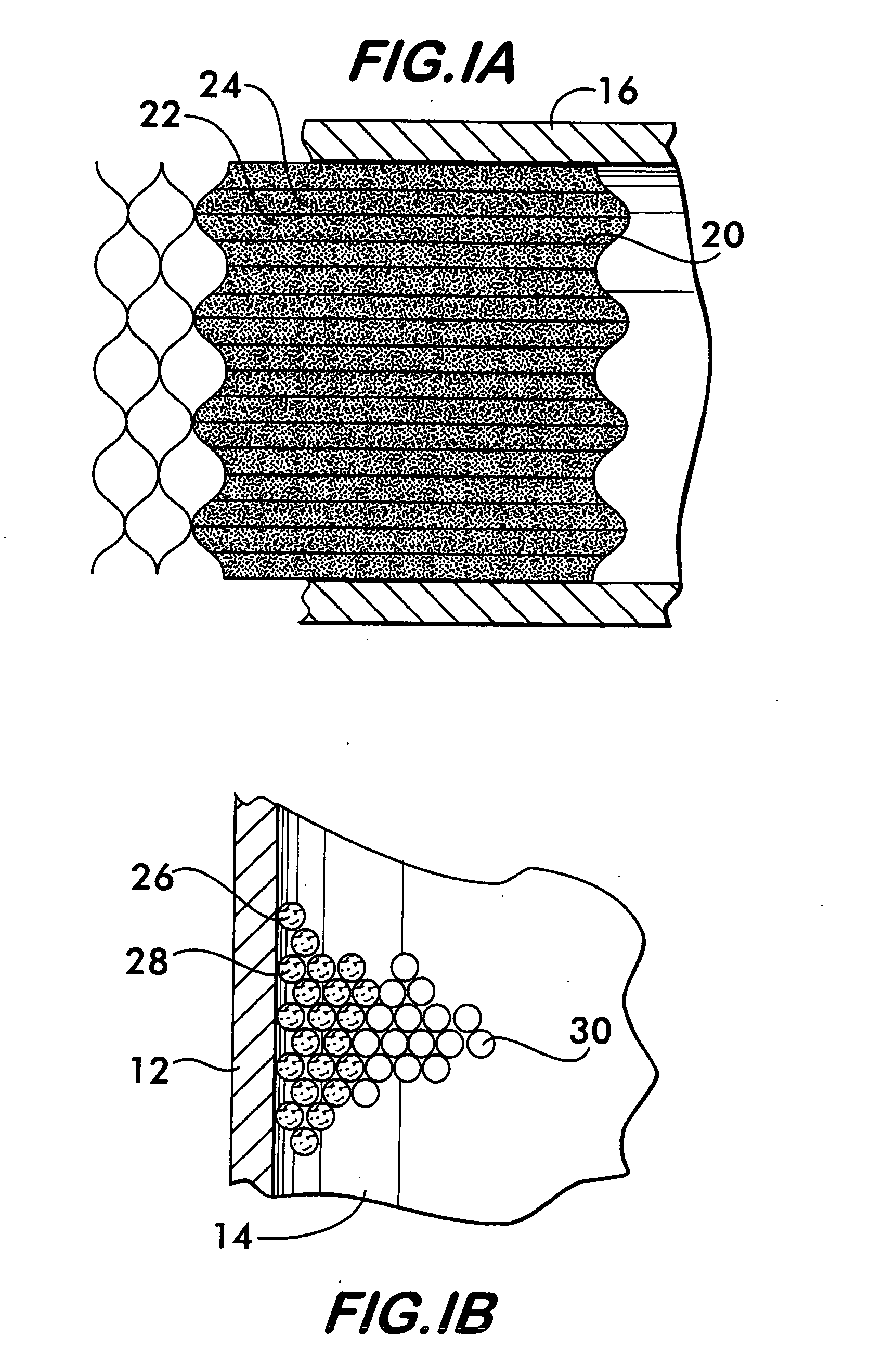
Thermally-integrated low temperature water-gas shift reactor apparatus and process
InactiveUS7074373B1Reduced toleranceExtended temperature rangeHydrogenPhysical/chemical process catalystsWater-gas shift reactionProduct gas
A thermally-integrated lower temperature water-gas shift reactor apparatus for converting carbon monoxide in the presence steam comprises a catalyst bed that is disposed within an outer region surrounding a waste heat recovery steam generator operating at a selected pressure corresponding to the optimum temperature for conducting the catalytic water-gas shift reaction and a process for useful recovery of the exothermic heat of reaction to generate steam that is used in a process for the conversion of hydrocarbon feedstock into useful gases such as hydrogen.
Owner:HARVEST ENERGY TECH
Hydrogen production method
ActiveUS7931888B2Maximize productionSteam flow rate essentially constantHydrogenCombustible gas catalytic treatmentMethane reformerMethane
A method of producing a hydrogen product stream in which a steam stream is reacted with a hydrocarbon containing stream within a steam methane reformer. The resulting product stream is subjected to a water gas shift reaction and then to pressure swing adsorption to produce the hydrogen product stream. The hydrocarbon stream is alternatively formed from a first type of feed stream made up of natural gas, refinery off-gas, naphtha or synthetic natural gas or combinations thereof and a second type that is additionally made up of a hydrogen and carbon monoxide containing gas. During use of both of the types of feed streams, the flow rate of the steam stream is not substantially changed and reformer exit temperatures of both the reactant and the flue gas side are held essentially constant.
Owner:PRAXAIR TECH INC
Carbon dioxide production method
ActiveUS20070232706A1Increased hydrogen recoveryPromote recoverySolidificationLiquefactionVacuum pressureCarbon dioxide production
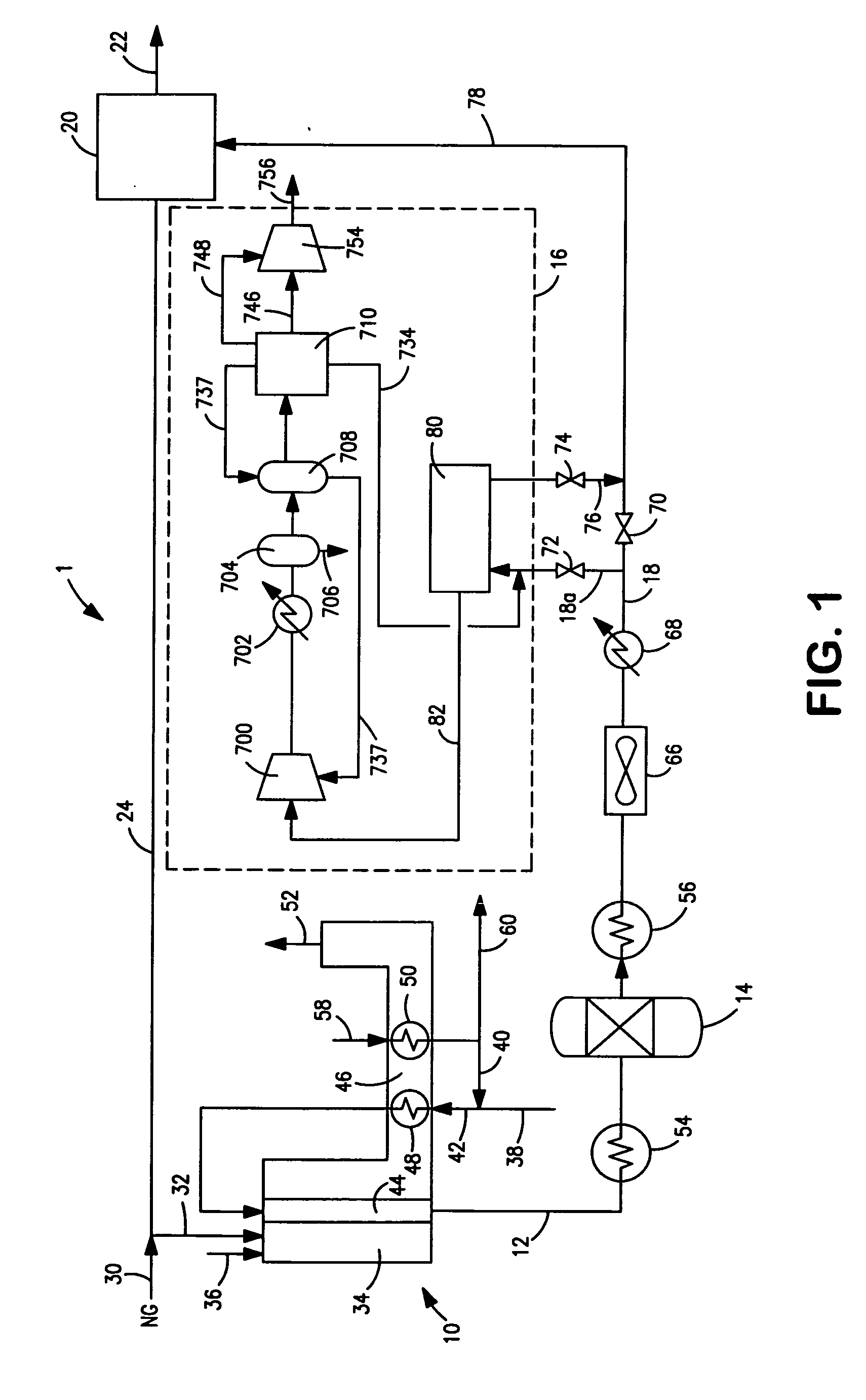
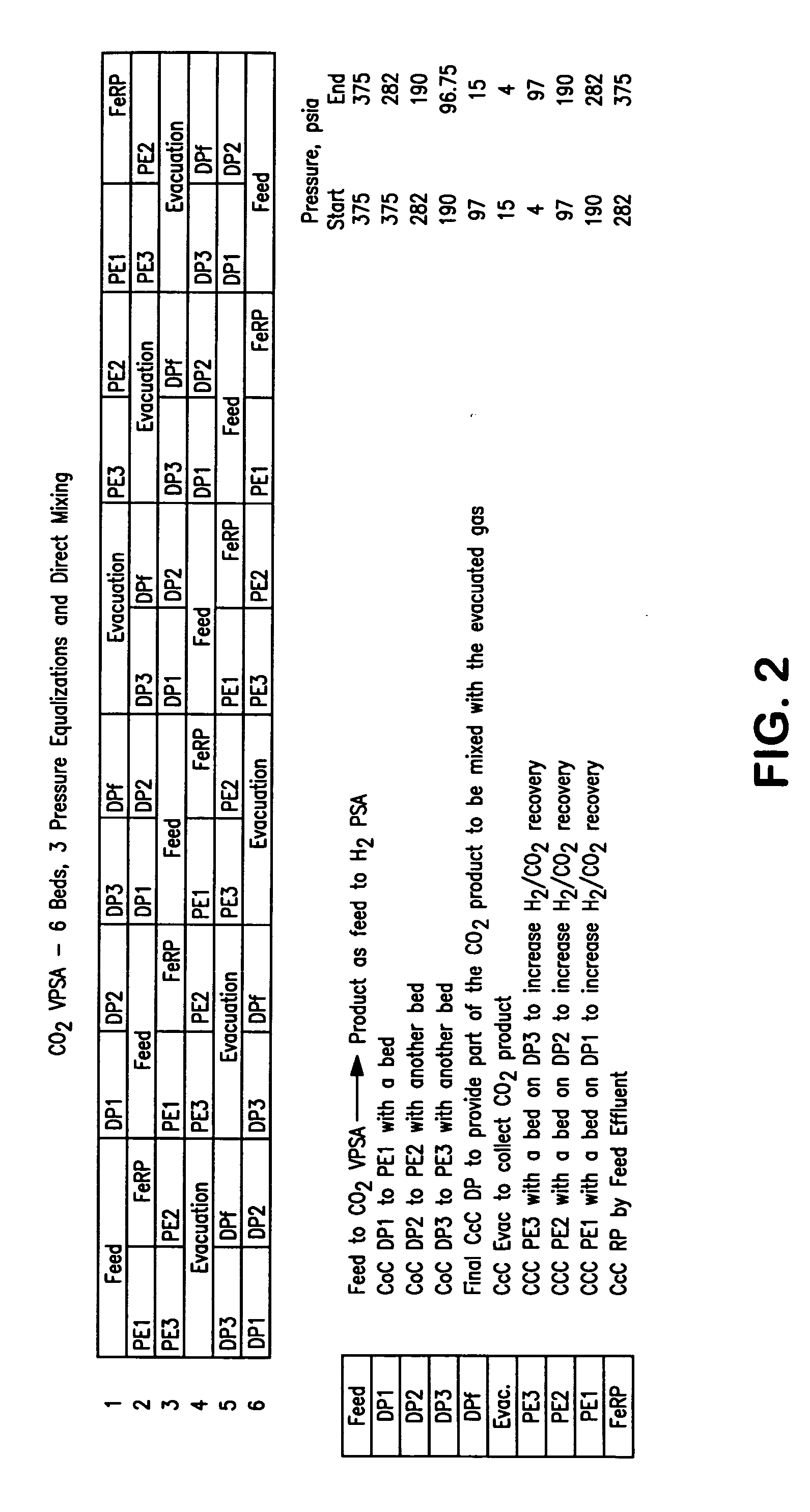
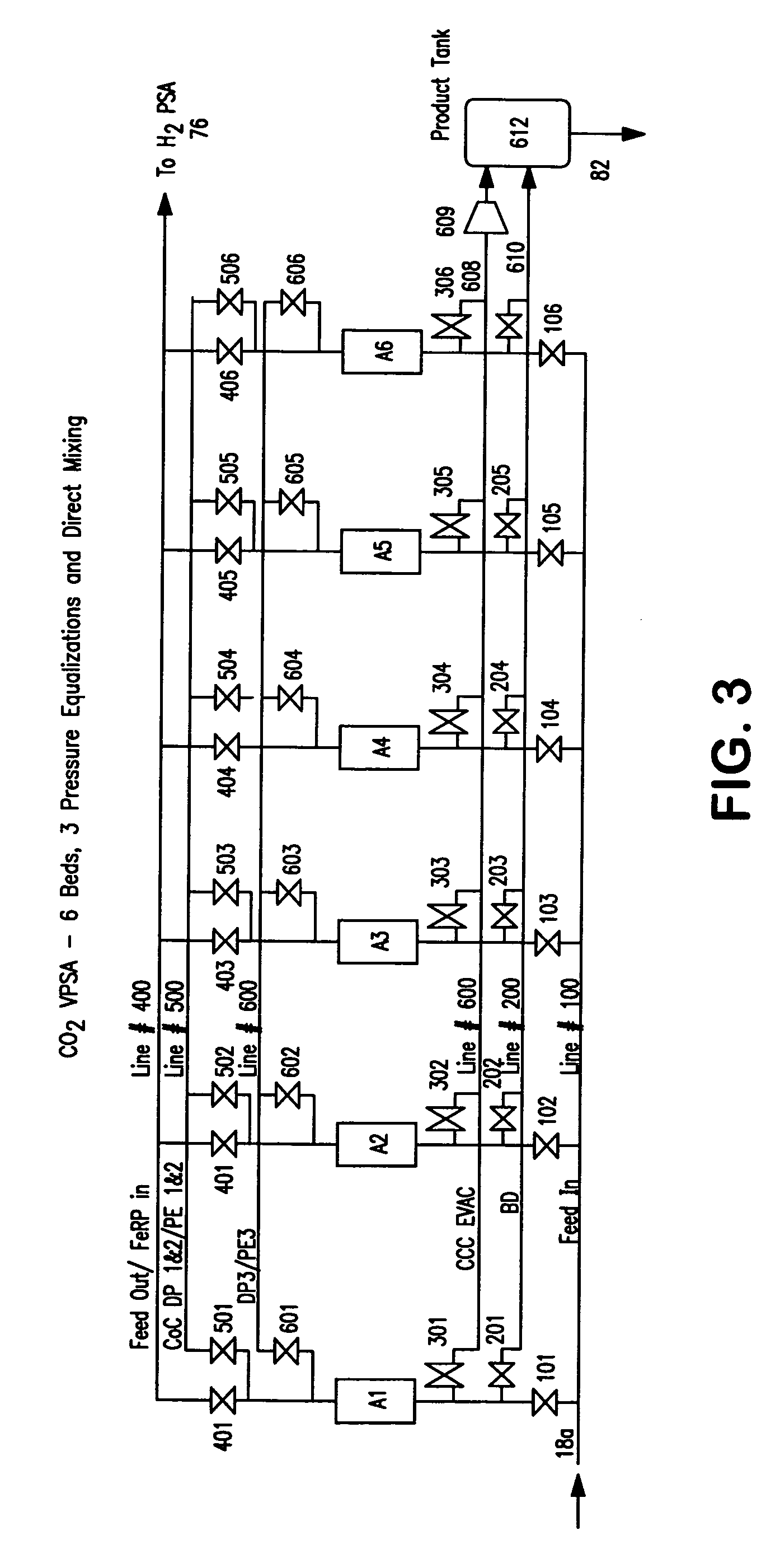
A method of producing a carbon dioxide product stream from a synthesis gas stream formed within a hydrogen plant having a synthesis gas reactor, a water-gas shift reactor, located downstream of the synthesis gas reactor to form the synthesis gas stream and a hydrogen pressure swing adsorption unit to produce a hydrogen product recovered from the synthesis gas stream. In accordance with the method the carbon dioxide from the synthesis gas stream by separating the carbon dioxide from the synthesis gas stream in a vacuum pressure swing adsorption system, thereby to produce a hydrogen-rich synthesis gas stream and a crude carbon dioxide stream and then purifying the crude carbon dioxide stream by a sub-ambient temperature distillation process thereby to produce the carbon dioxide product. A hydrogen synthesis gas feed stream to the hydrogen pressure swing adsorption unit is formed at least in part from the hydrogen rich stream.
Owner:PRAXAIR TECH INC
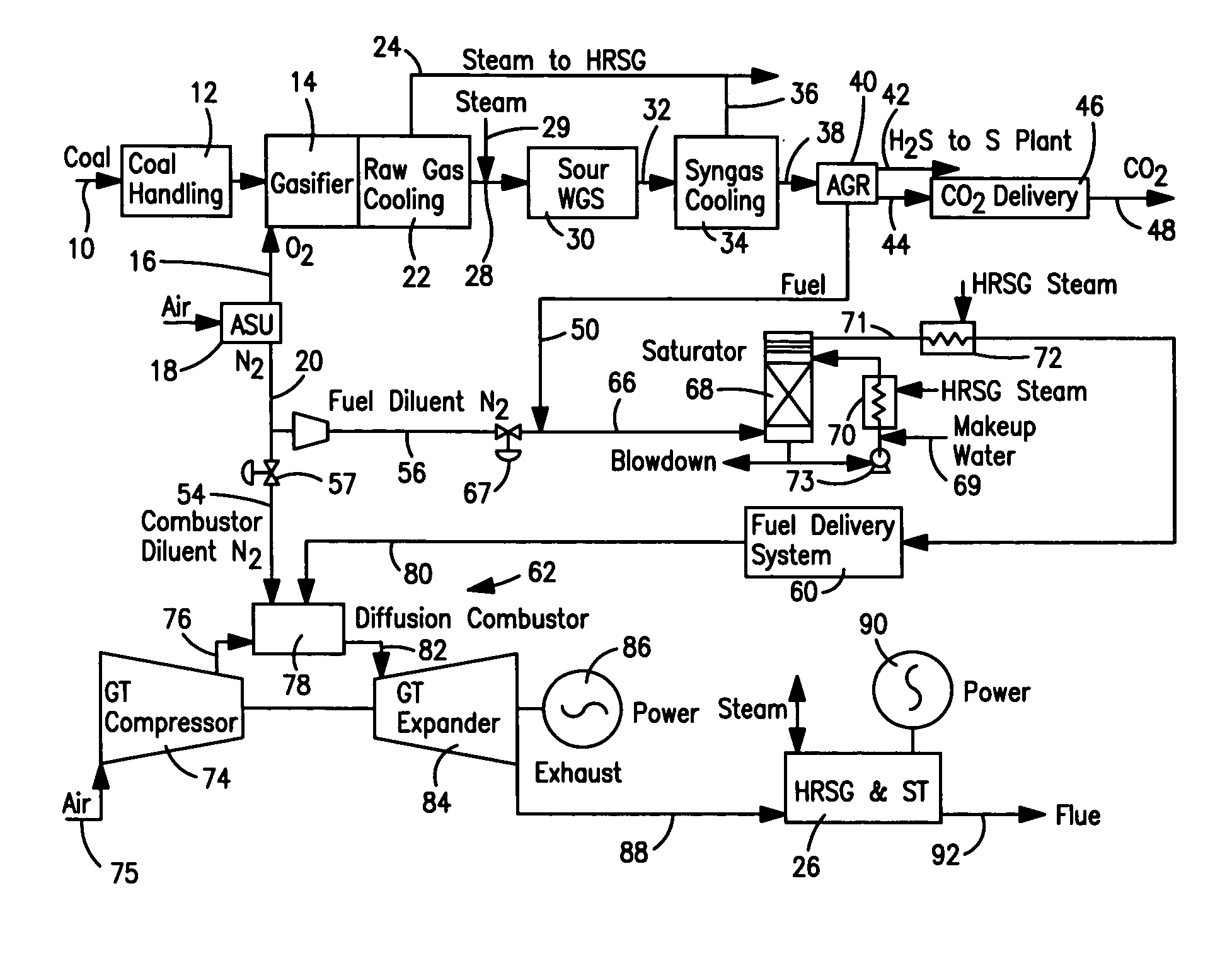
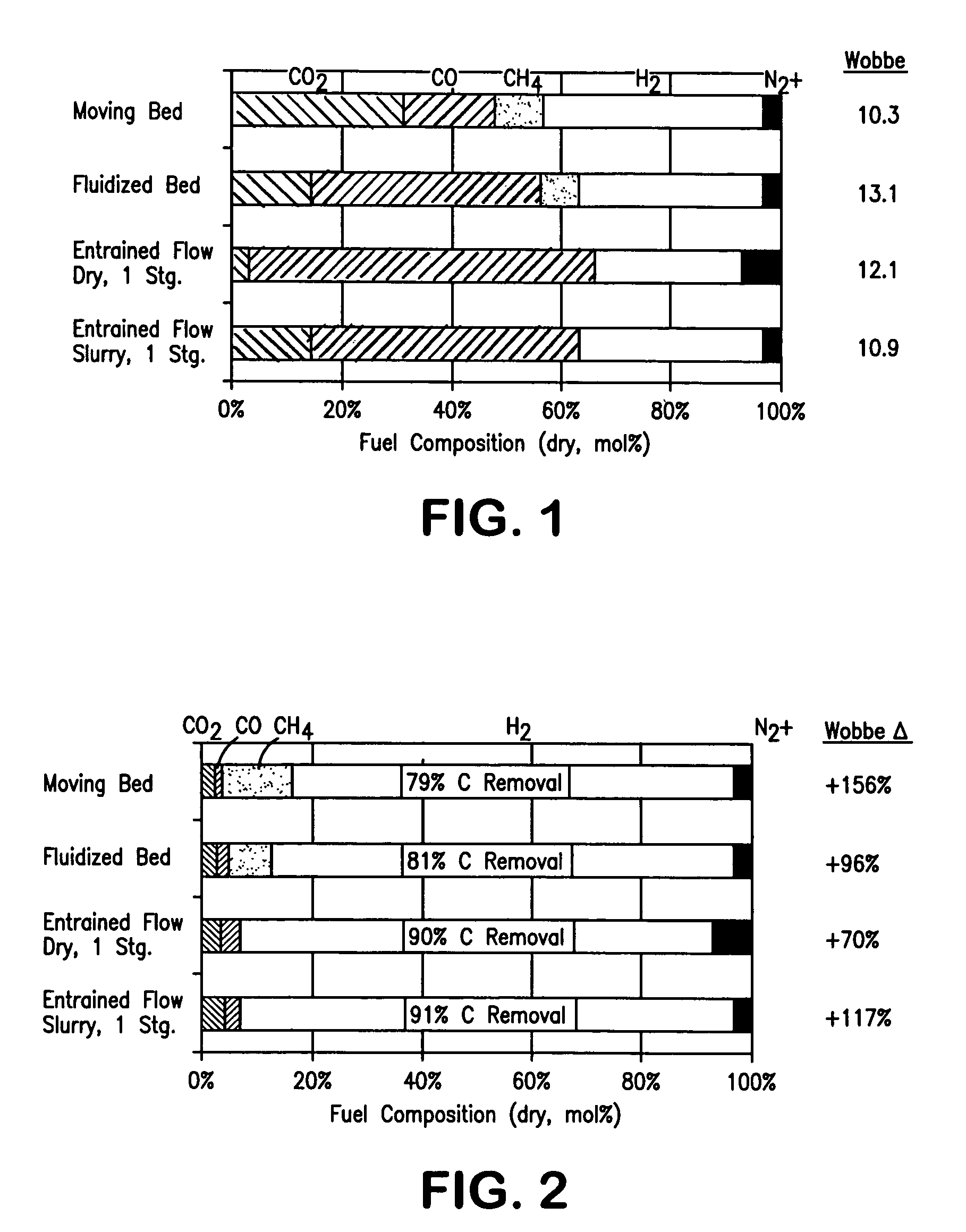
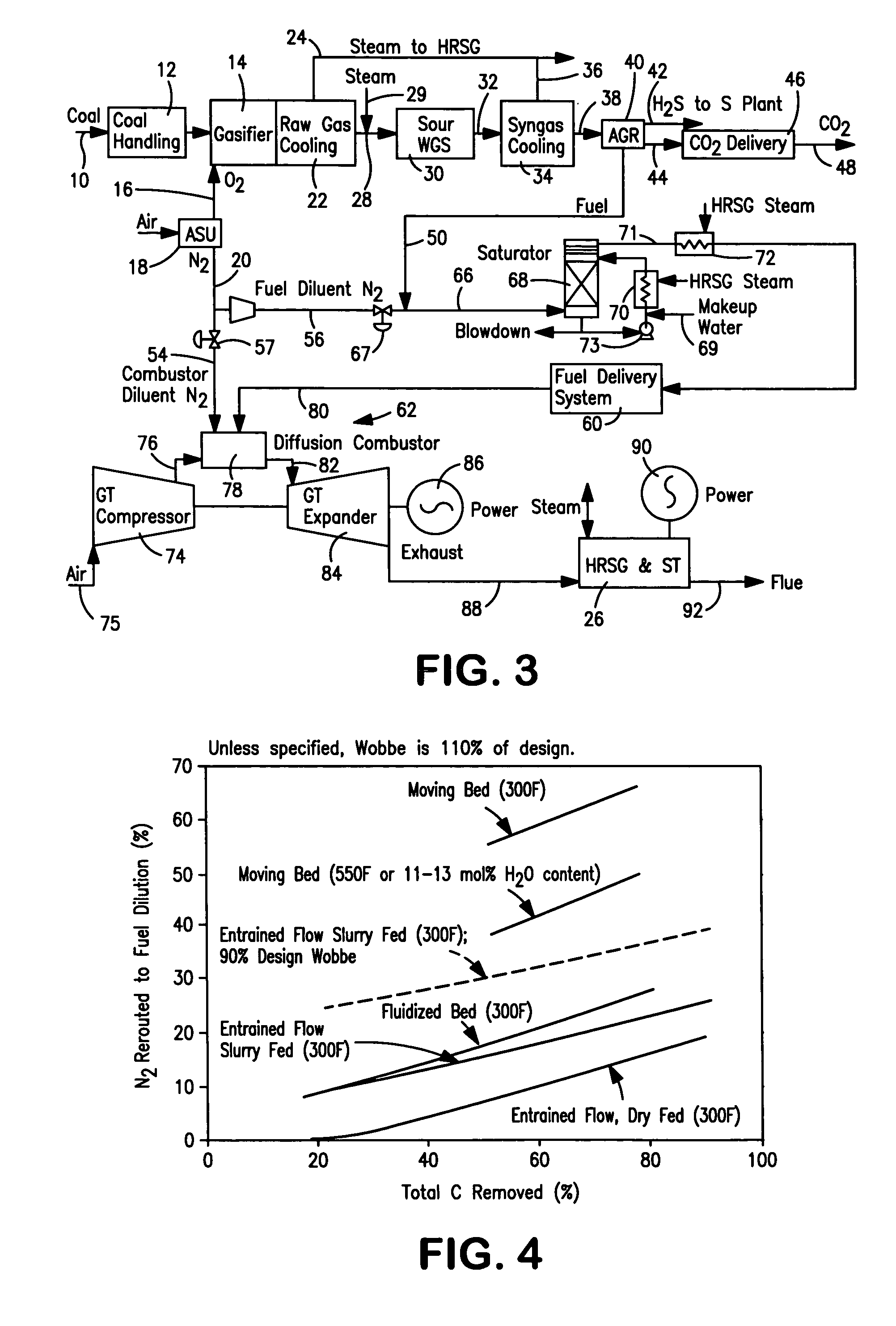
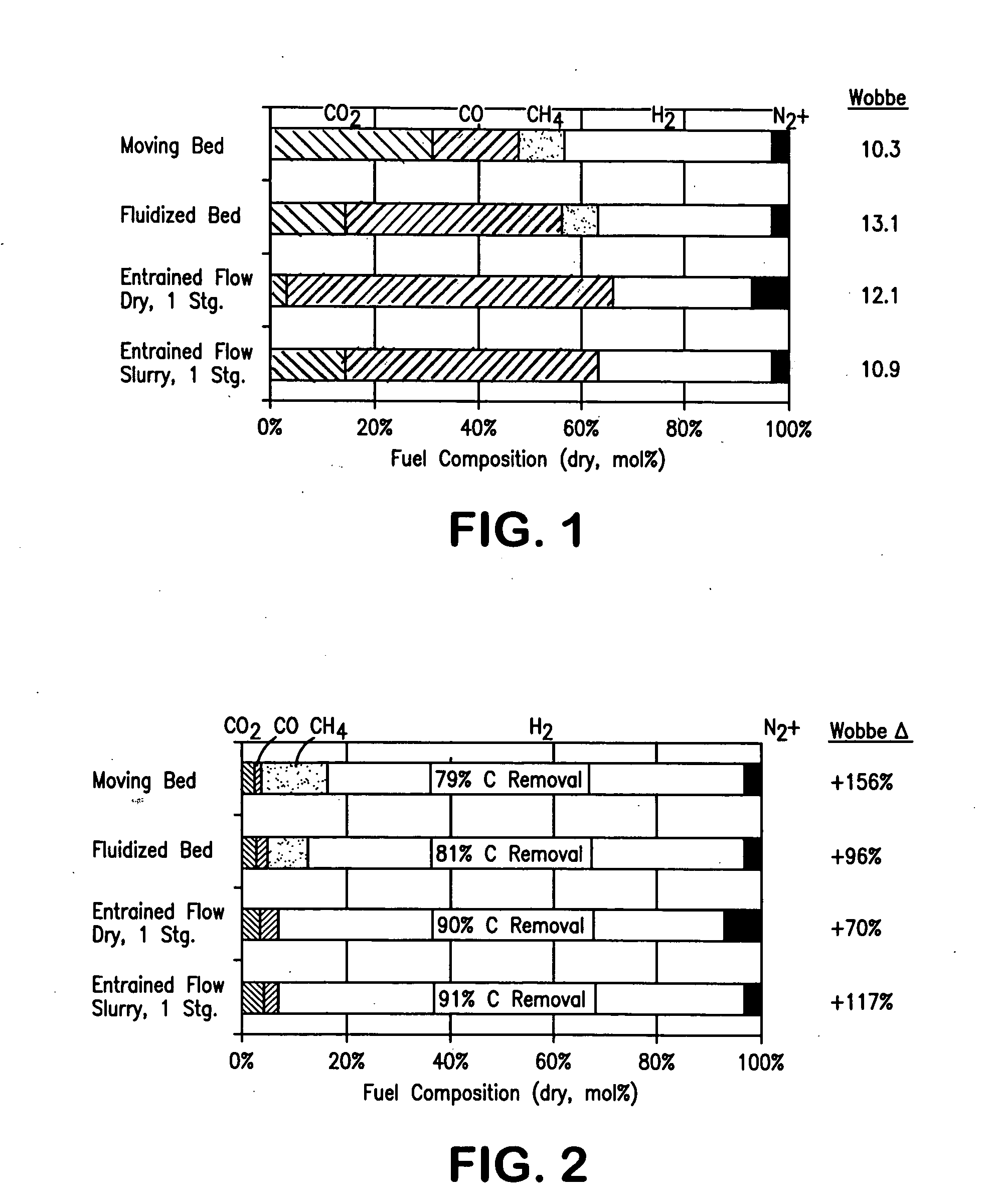
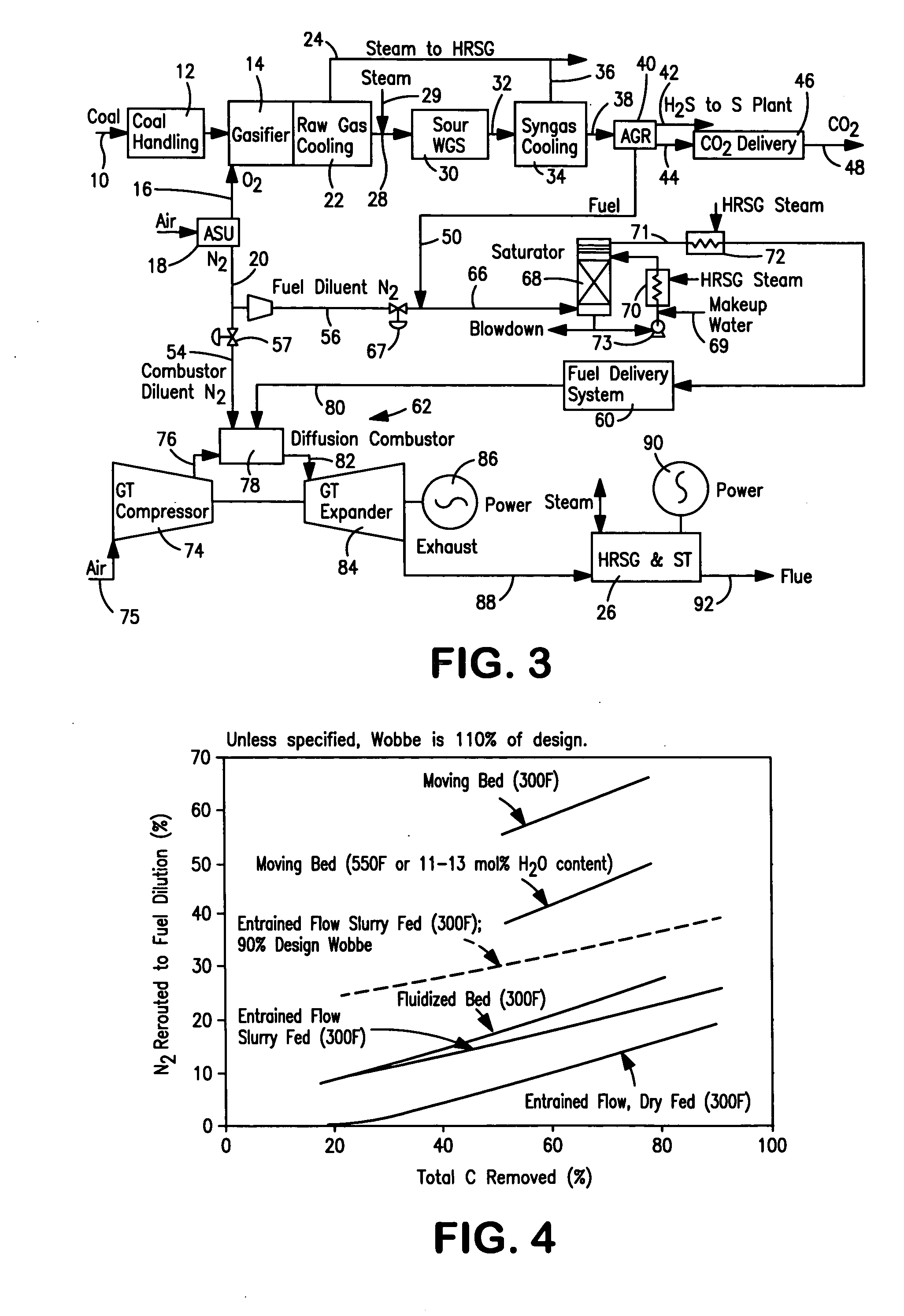
Method of maintaining a fuel Wobbe index in an IGCC installation
InactiveUS7690204B2Low indexFuel supply regulationGaseous fuel feeder/distributionWater-gas shift reactionNitrogen
A method of reducing a modified Wobbe index of a fuel stream fed to diffusion combustors of a gas turbine that is used in connection with an IGCC installation in which a nitrogen stream is fed into the head ends of the combustor of NOx control and the modified Wobbe index of the fuel stream has been increased in an amount that is greater than at least about 10 percent of a design Wobbe index for the fuel to be fed to the gas turbine. The reason for the increase is the conversion of the carbon monoxide within the fuel stream to hydrogen and carbon dioxide by a water gas shift reaction and subsequent removal of the carbon dioxide. The nitrogen stream is maintained at the same level both with and without conversion and subsequent removal of carbon atoms. The nitrogen stream is divided to subsidiary streams that are respectively fed into the head end of the combustors and that are mixed with a fuel to lower the modified Wobbe index to acceptable levels for the gas turbine.
Owner:PRAXAIR TECH INC
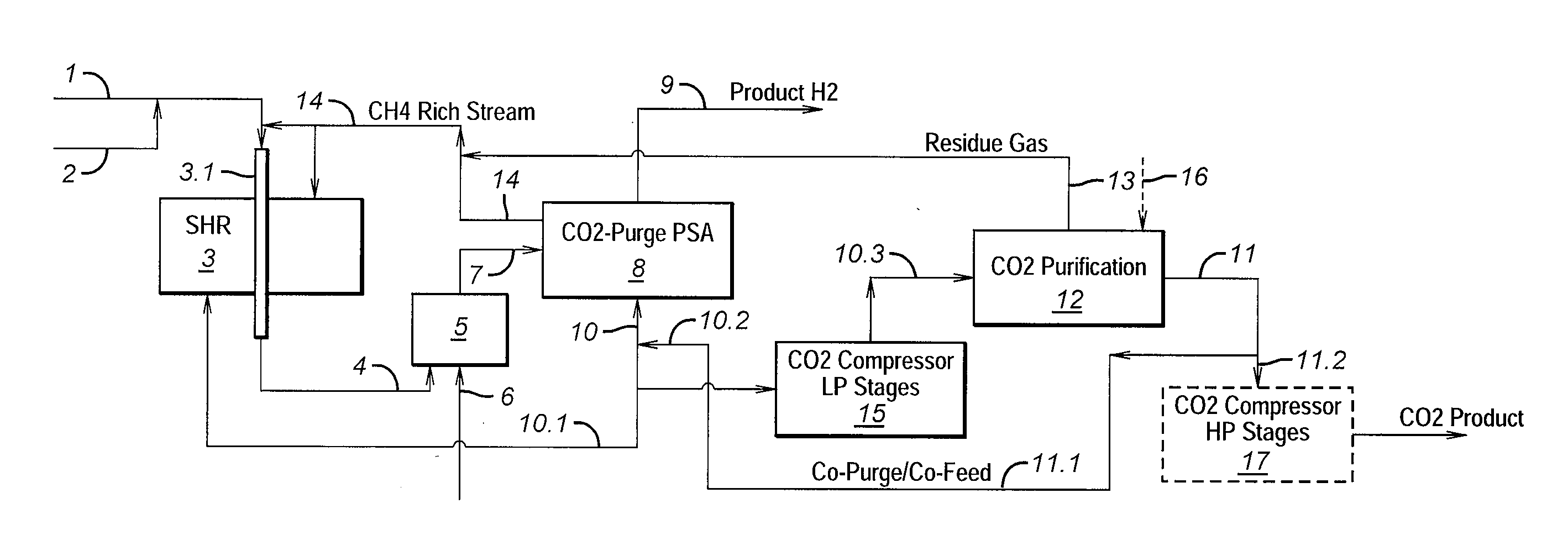
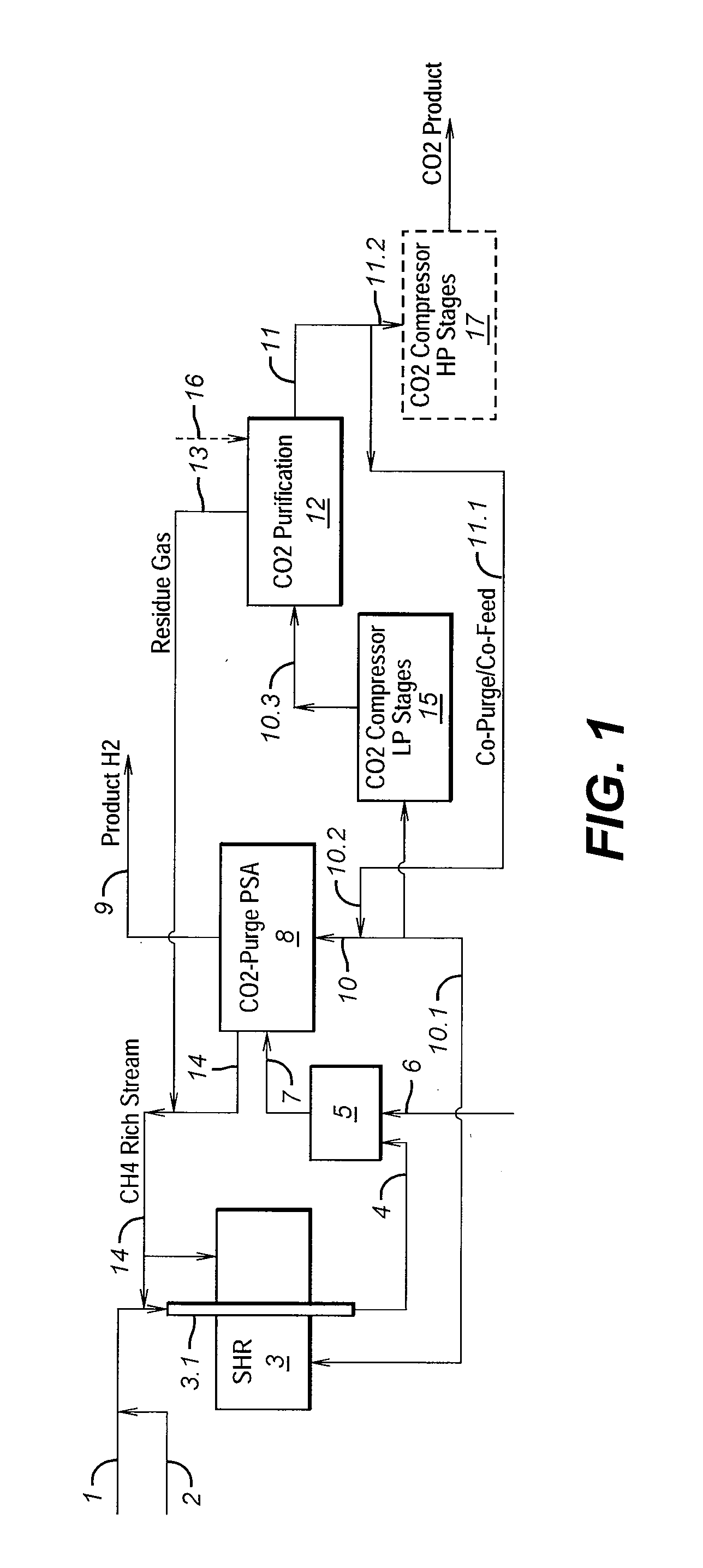
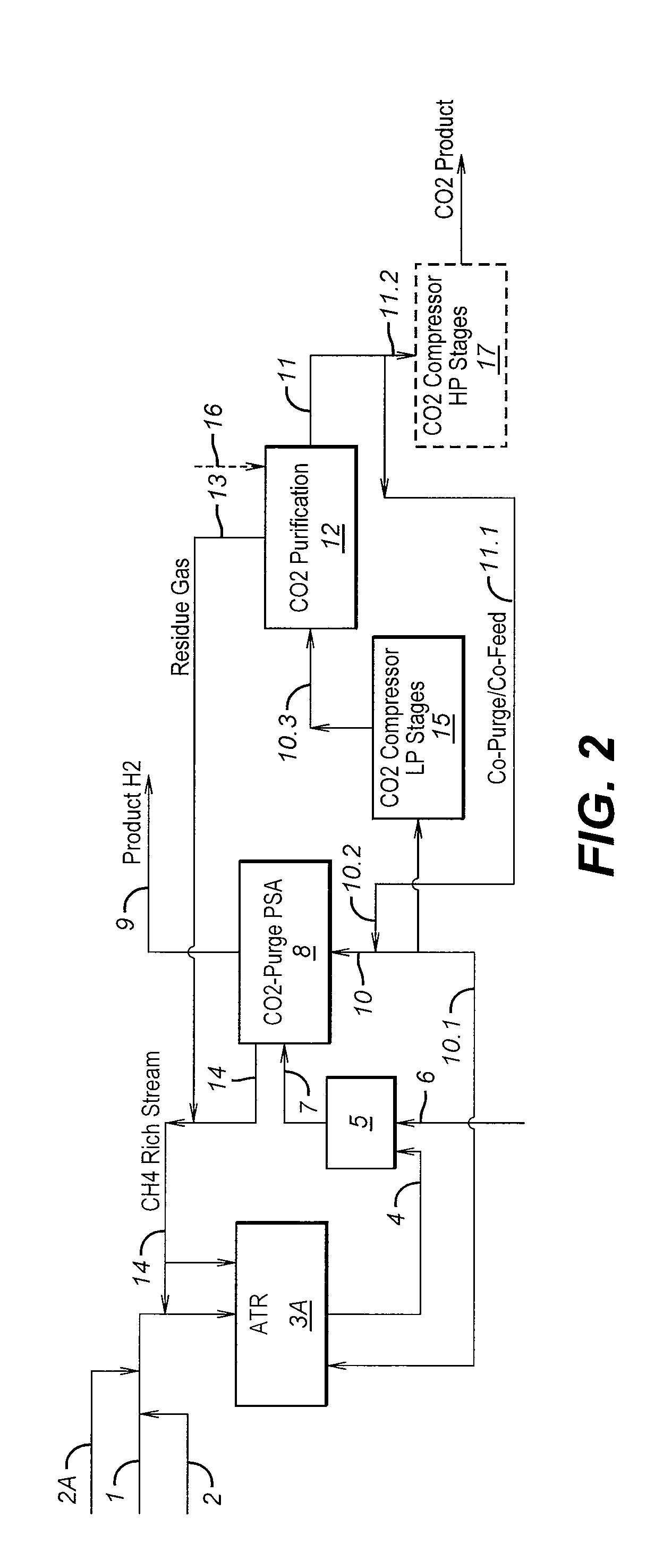
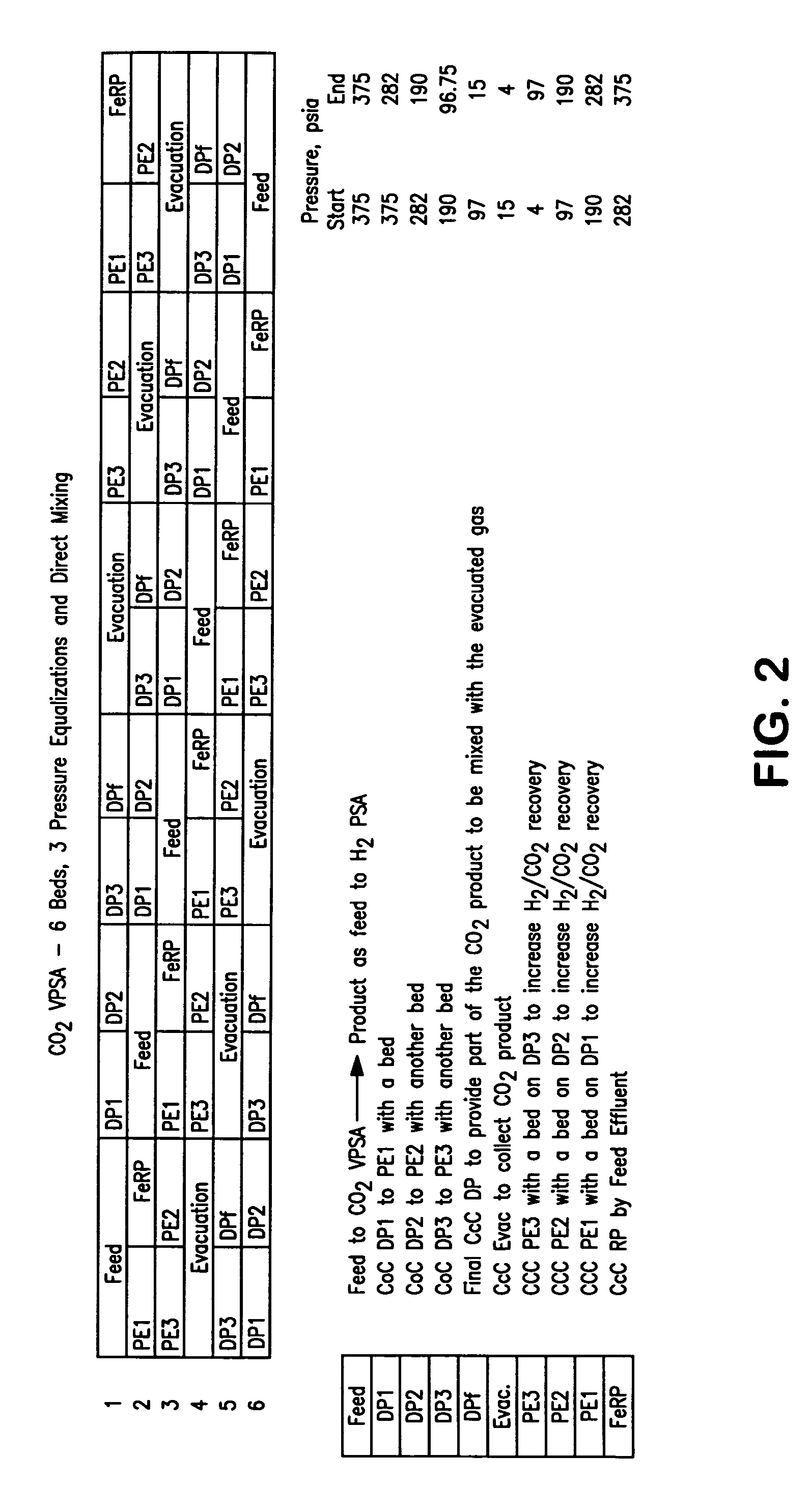
Process For The Production Of Carbon Dioxide Utilizing A Co-Purge Pressure Swing Adsorption Unit
The present invention provides a process for recovering gaseous hydrogen and gaseous carbon dioxide from a mixture of hydrocarbons by utilizing a system that includes a reformer unit, an optional water gas shift reactor, and a pressure swing adsorption unit in conjunction with a carbon dioxide purification unit such as a cryogenic purification unit or a catalytic oxidizer. In this process, purified CO2 from the CO2 purification unit is used as a co-feed / co-purge in the pressure swing adsorption unit in order to produce a CO2 tail gas that includes a higher concentration of CO2.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Catalytic method to remove CO and utilize its energy content in CO-containing streams
InactiveUS20060024539A1Low costUse minimizedMaterial nanotechnologyGas treatmentFuel cellsCatalytic method
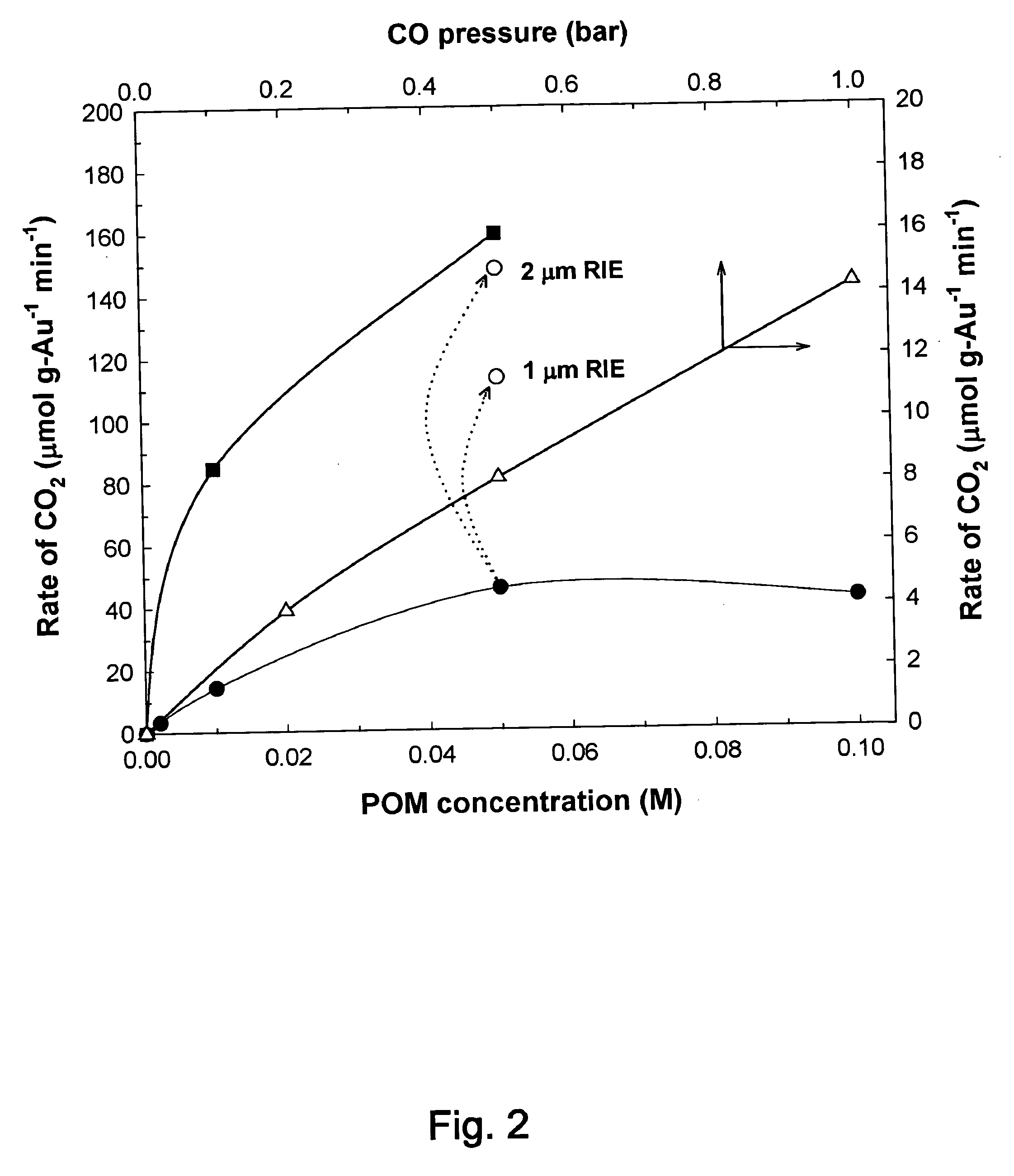
Disclosed are a reactor and a corresponding method for producing electrical energy using a fuel cell by selectively oxidizing CO at room temperature using polyoxometalate compounds and transition metal compounds over metal-containing catalysts, thereby eliminating the water-gas shift reaction and the need to transport and vaporize liquid water in the production of H2 for fuel cells. The reactor also functions to deplete CO from an incoming gas stream.
Owner:WISCONSIN ALUMNI RES FOUND
Hot solids gasifier with CO2 removal and hydrogen production
ActiveUS7083658B2Avoid entrainmentEfficient captureMuffle furnacesGas modification by gas mixingCo2 removalWater-gas shift reaction
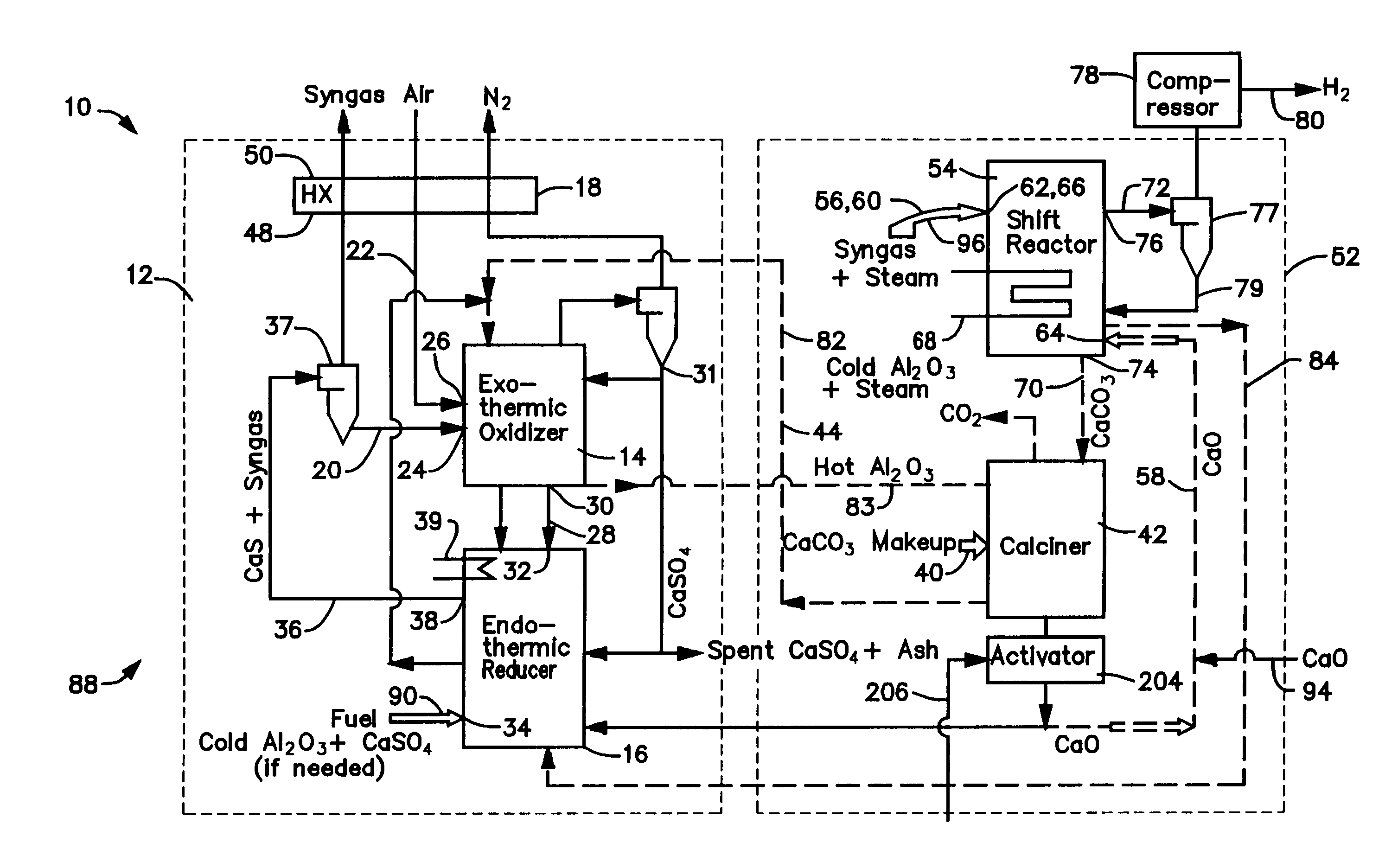
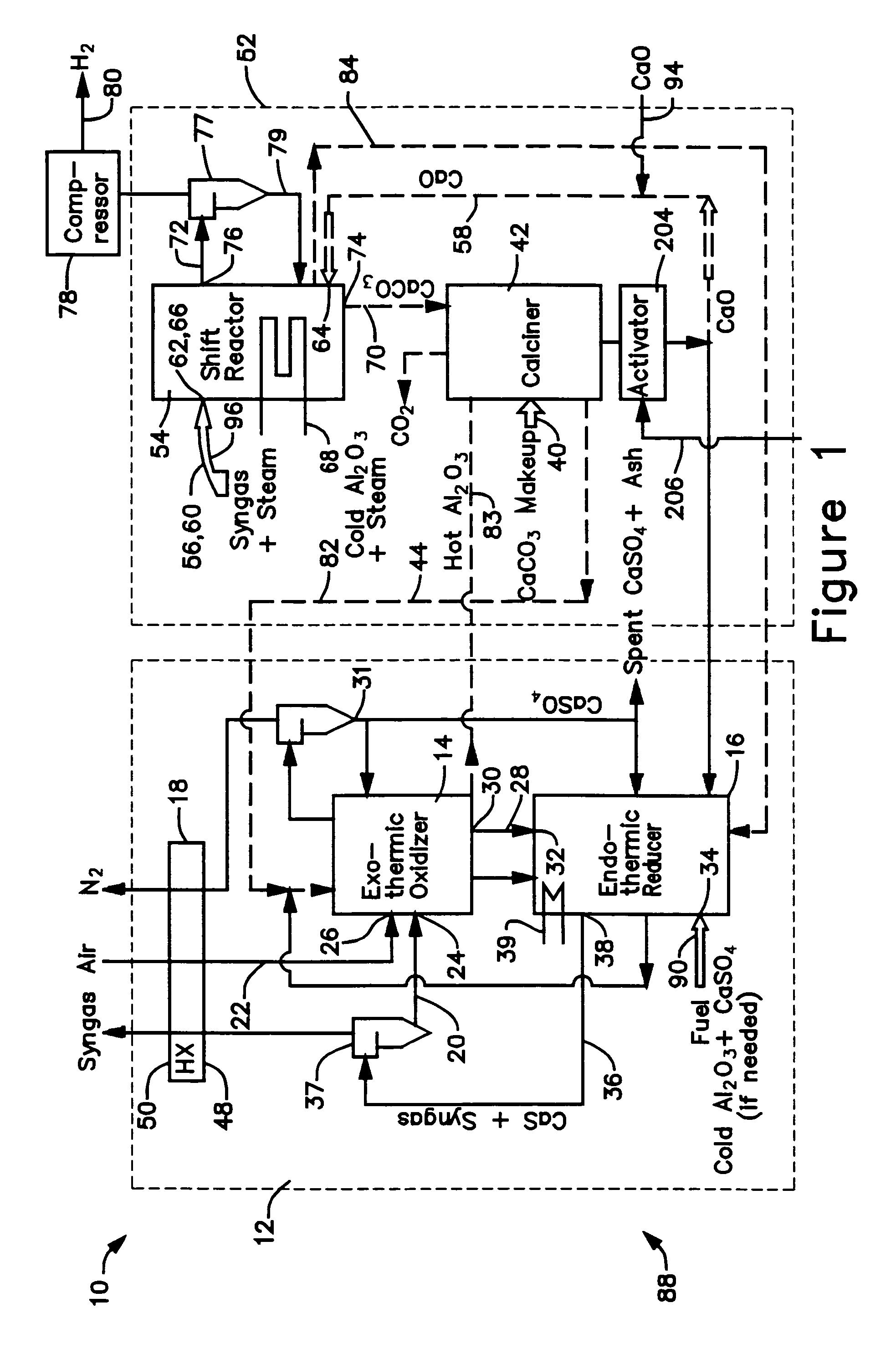
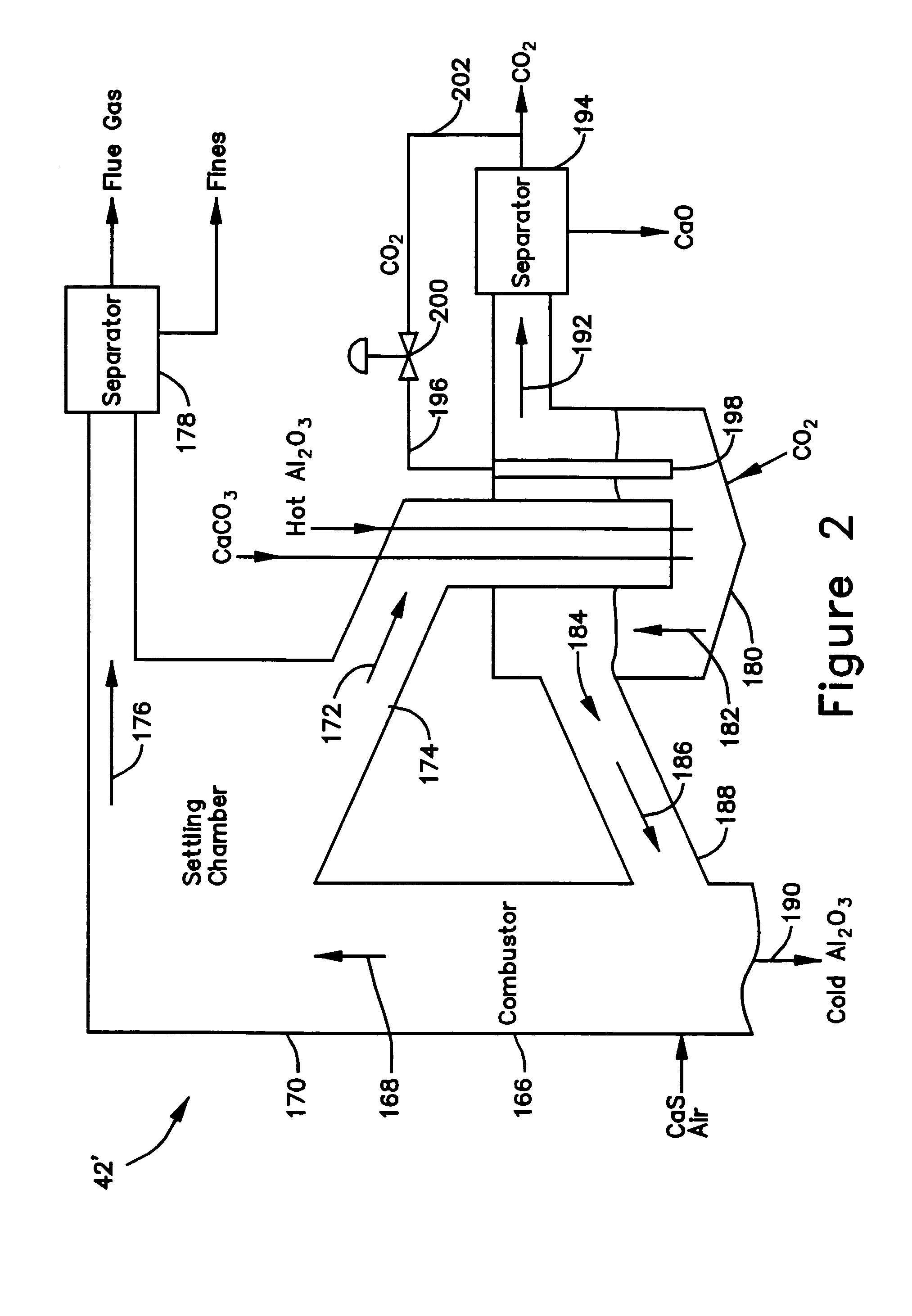
A gasifier 10 includes a first chemical process loop 12 having an exothermic oxidizer reactor 14 and an endothermic reducer reactor 16. CaS is oxidized in air in the oxidizer reactor 14 to form hot CaSO4 which is discharged to the reducer reactor 16. Hot CaSO4 and carbonaceous fuel received in the reducer reactor 16 undergo an endothermic reaction utilizing the heat content of the CaSO4, the carbonaceous fuel stripping the oxygen from the CaSO4 to form CaS and a CO rich syngas. The CaS is discharged to the oxidizer reactor 14 and the syngas is discharged to a second chemical process loop 52. The second chemical process loop 52 has a water-gas shift reactor 54 and a calciner 42. The CO of the syngas reacts with gaseous H2O in the shift reactor 54 to produce H2 and CO2. The CO2 is captured by CaO to form hot CaCO3 in an exothermic reaction. The hot CaCO3 is discharged to the calciner 42, the heat content of the CaCO3 being used to strip the CO2 from the CaO in an endothermic reaction in the calciner, with the CaO being discharged from the calciner 42 to the shift reactor 54.
Owner:AIR PROD & CHEM INC +1
Producing Liquid Fuel from Organic Material such as Biomass and Waste Residues
InactiveUS20140224706A1Combustible gas catalytic treatmentGas modification by gas mixingWaxForming gas
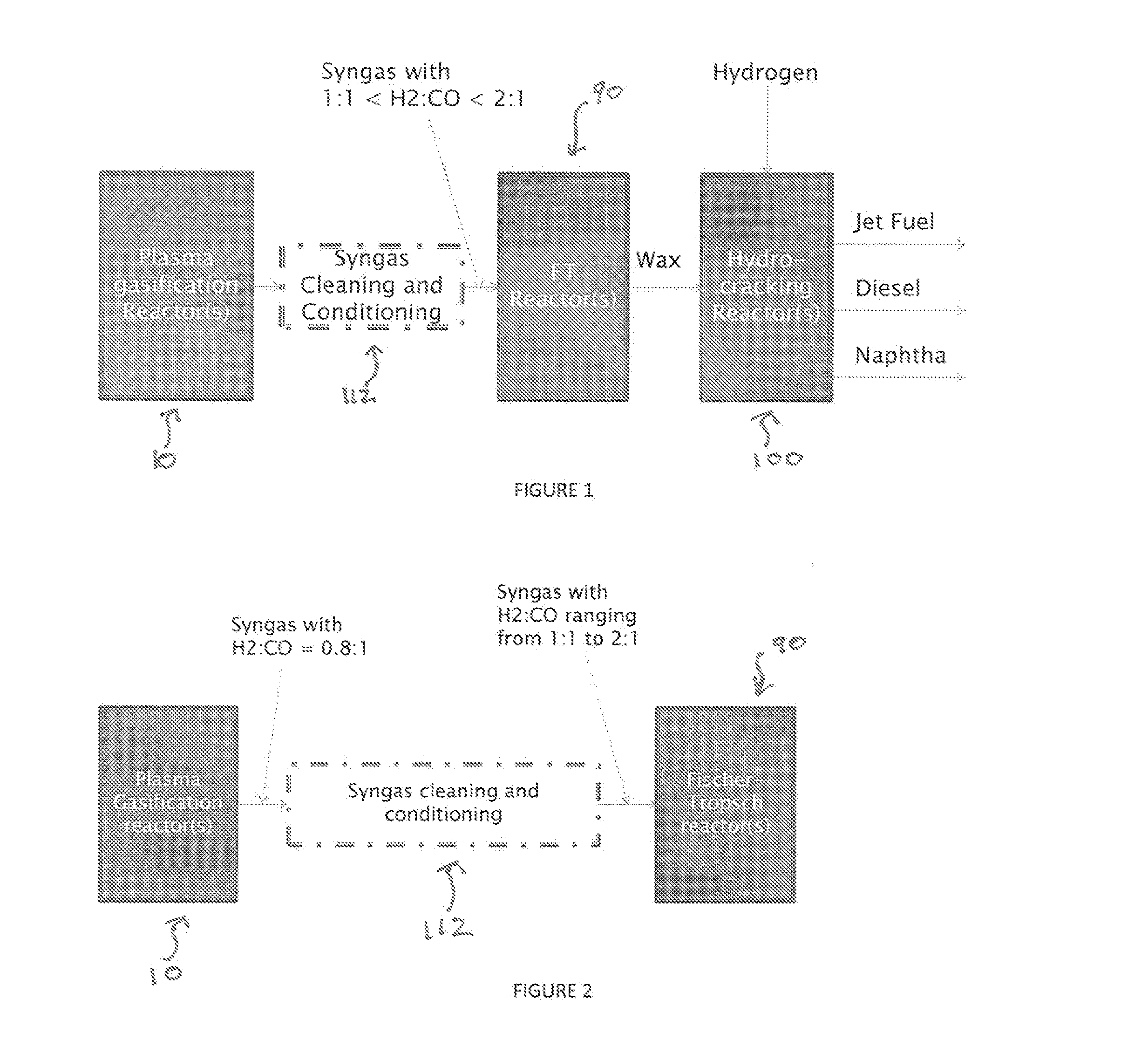
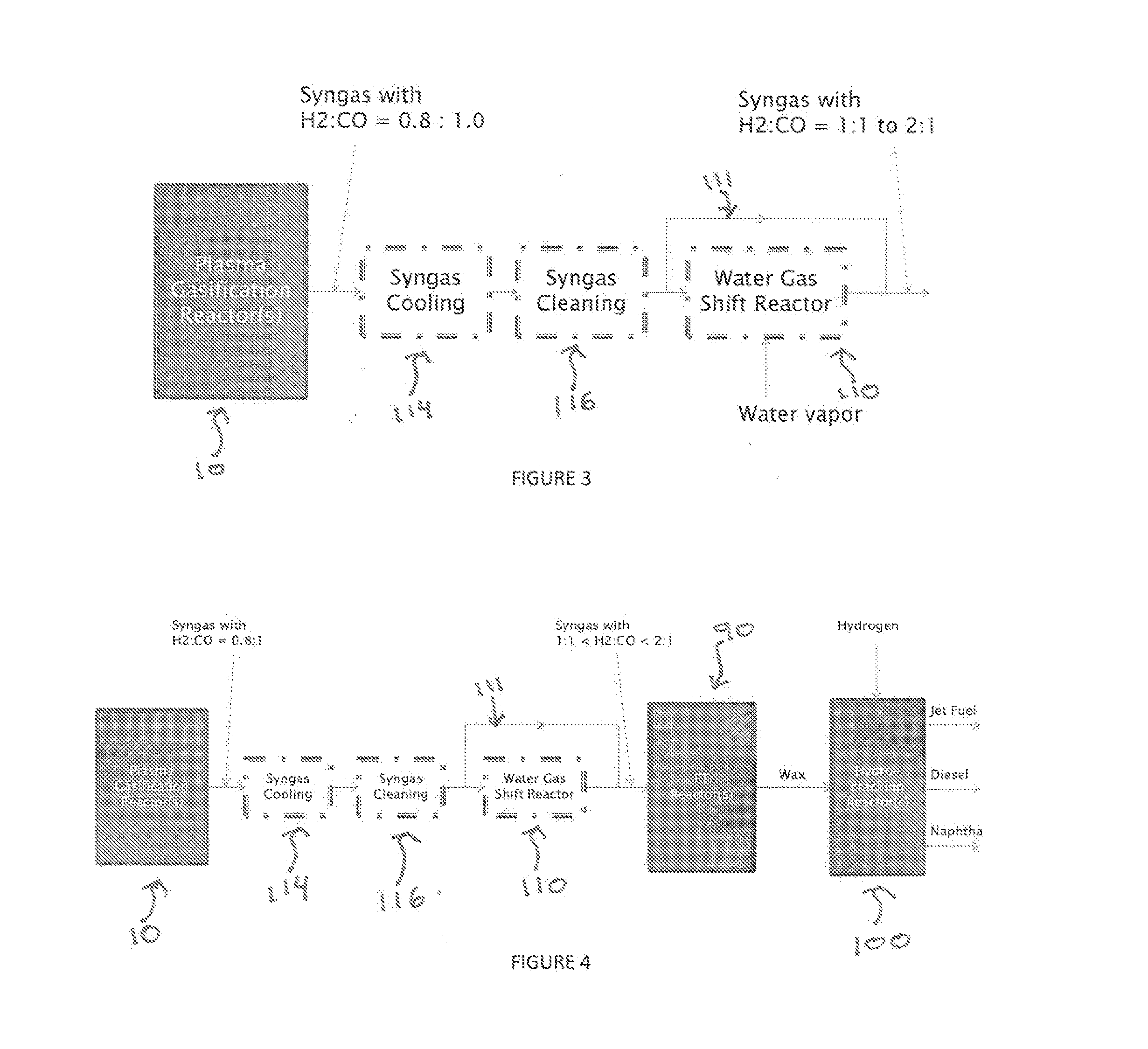
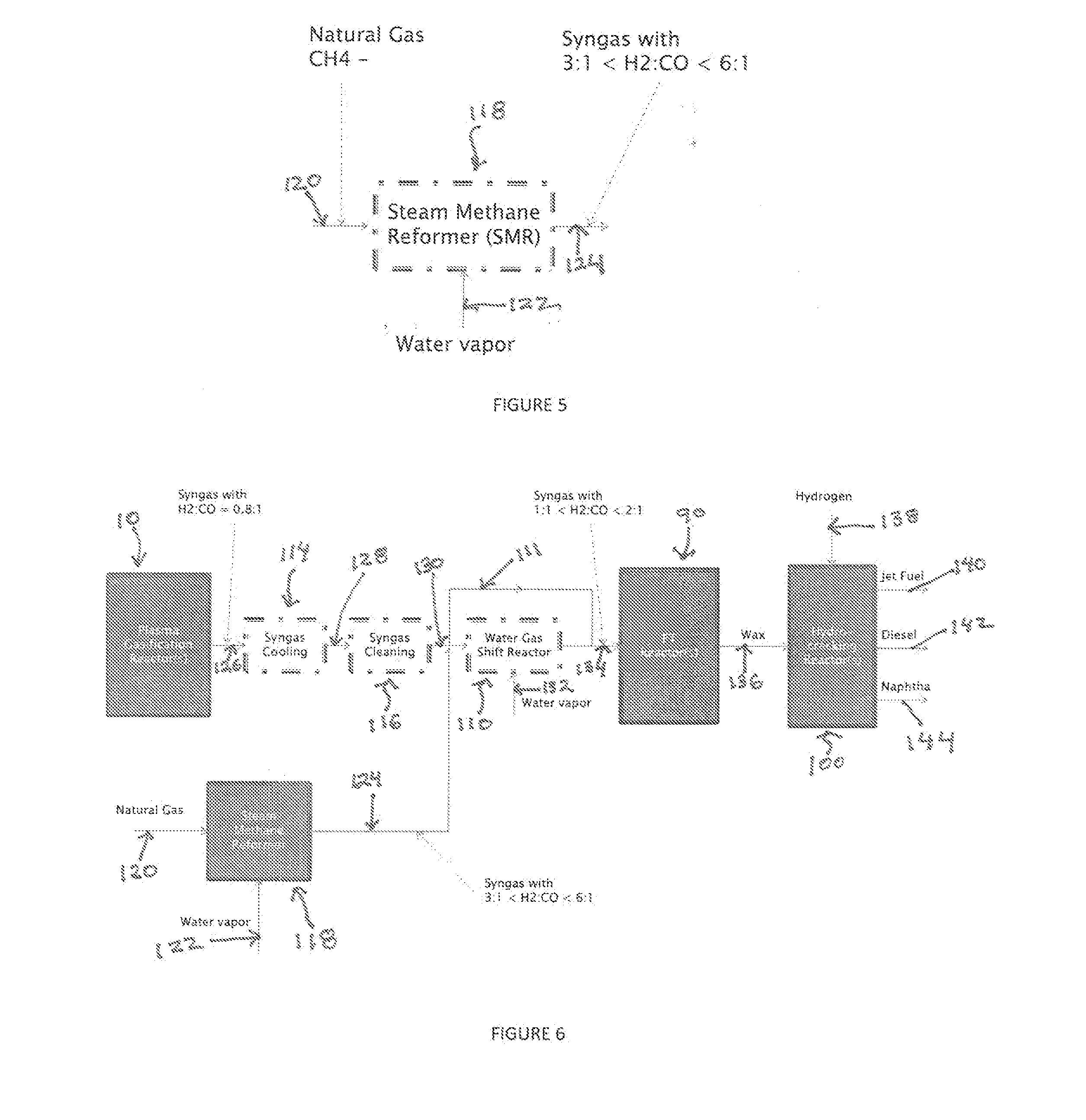
A process for producing liquid fuel from biomass feed stock comprising feeding a biomass feedstock into a one stage atmospheric pressure thermo-catalytic plasma gasifier, contacting the feedstock with oxygen or steam or both to obtain a syngas stream; splitting the syngas stream into first and second streams; conveying the first stream to a water gas shift reactor for producing a modified syngas stream containing CO and hydrogen; the second stream bypassing the water gas shift reactor and being added to the modified syngas steam; optionally reforming natural gas by steam methane reforming to produce a synthetic gas and optionally adding the synthetic gas to the water gas shift reactor; thereby obtaining a syngas having a H2:CO ratio of about 1:1 to about 2:1; subjecting the syngas to a Fischer Tropsch reaction thereby producing a wax product; and subjecting the product to a hydrogen cracking process to produce liquid fuel; and apparatus therefore.
Owner:SOLENA FUELS CORP
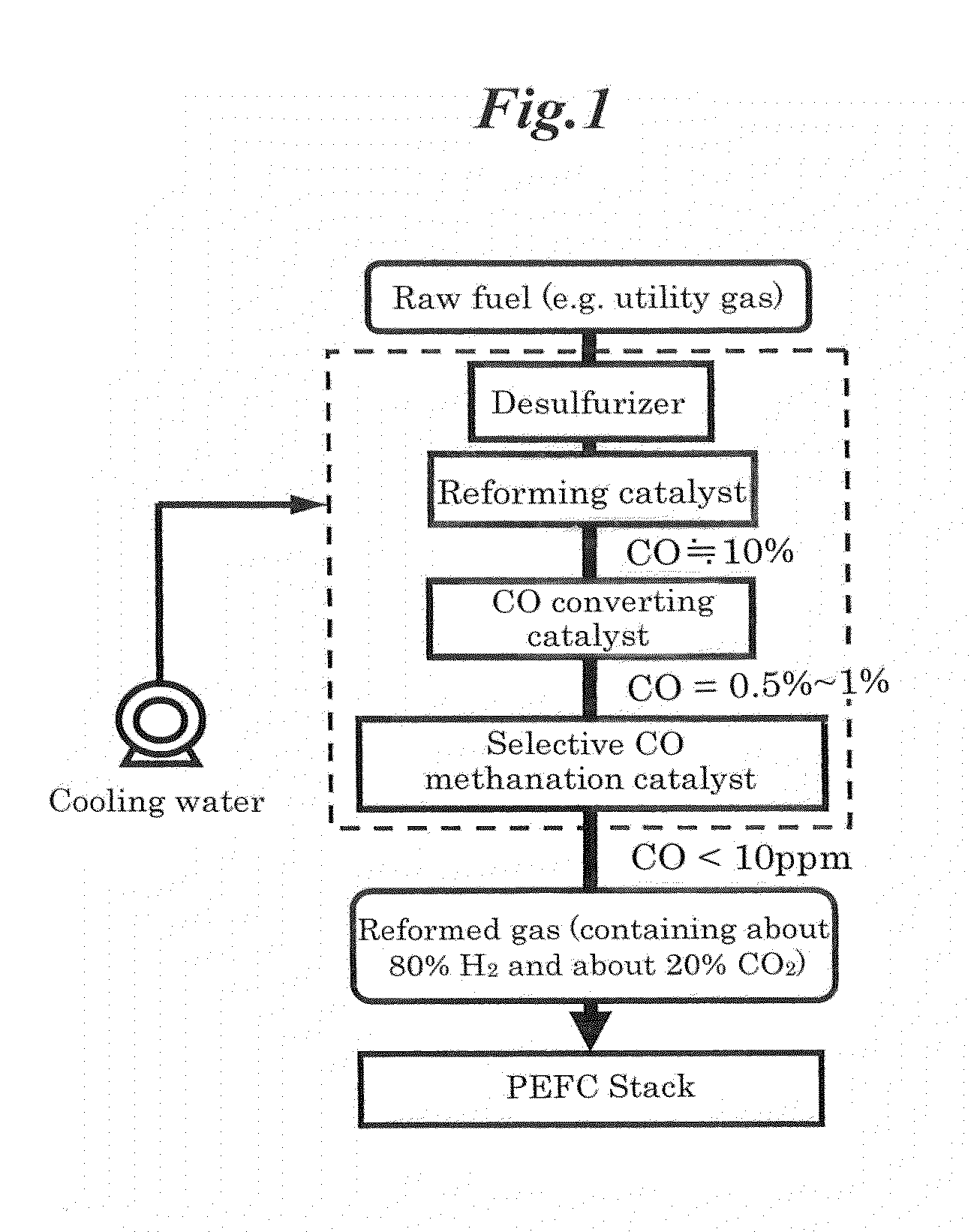
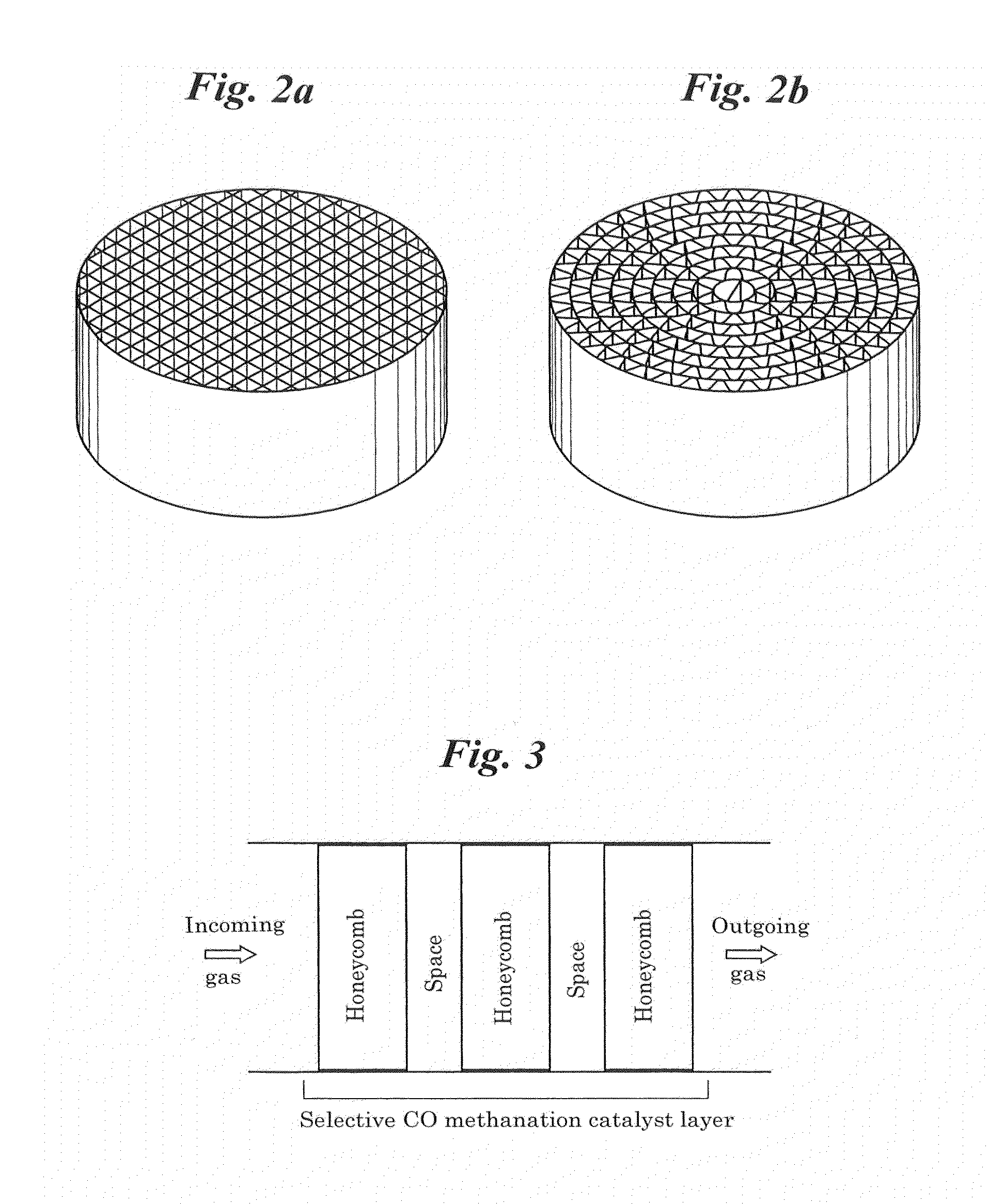
Selective co methanation catalyst, method of producing the same, and apparatus using the same
ActiveUS20120063963A1High low temperature activityReduce supportHydrogenFinal product manufactureReduction treatmentMethanation
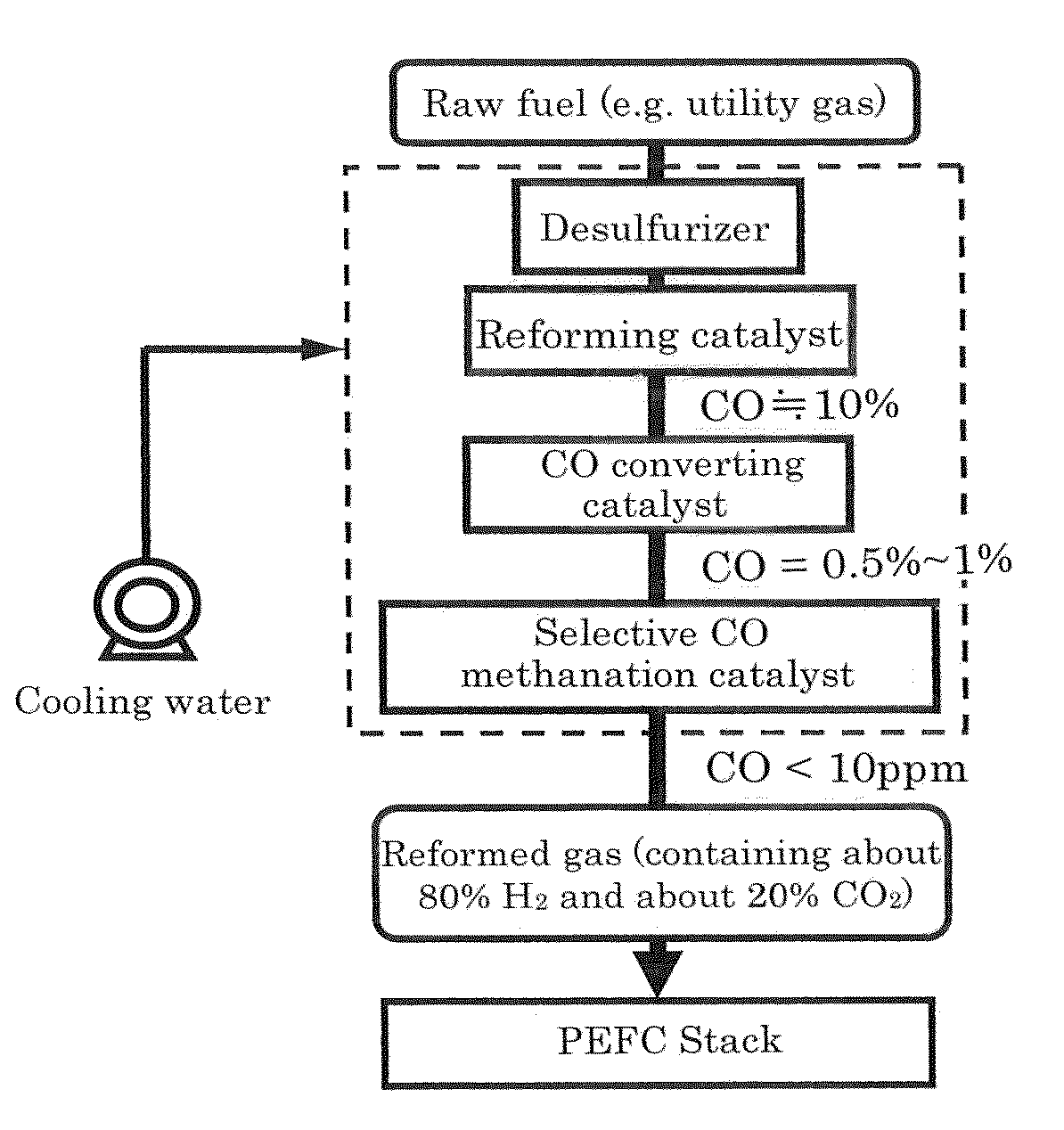
Provided is a new catalyst capable of removing carbon monoxide economically without adding particular reaction gas externally. Also provided are a process for producing and an apparatus using such a catalyst. Impregnation of a Ni—Al composite oxide precursor of a nonstoichiometric composition prepared by the solution-spray plasma technique with a ruthenium salt to be supported and performing reduction treatment allows CO methanation reaction to selectively proceed even in the high-temperature range in which CO2 methanation reaction and reverse water-gas-shift reaction proceed preferentially with conventional catalysts. Selective CO methanation reaction occurs reproducibly with another Ni—Al composite oxide precursor or an additive metallic species. Also, the low-temperature activity of CO methanation reaction can be improved through steps different from conventional catalyst production processes in producing such a catalyst material, whereby the temperature window the resulting catalyst material has can be utilized most effectively.
Owner:UNIVERSITY OF YAMANASHI
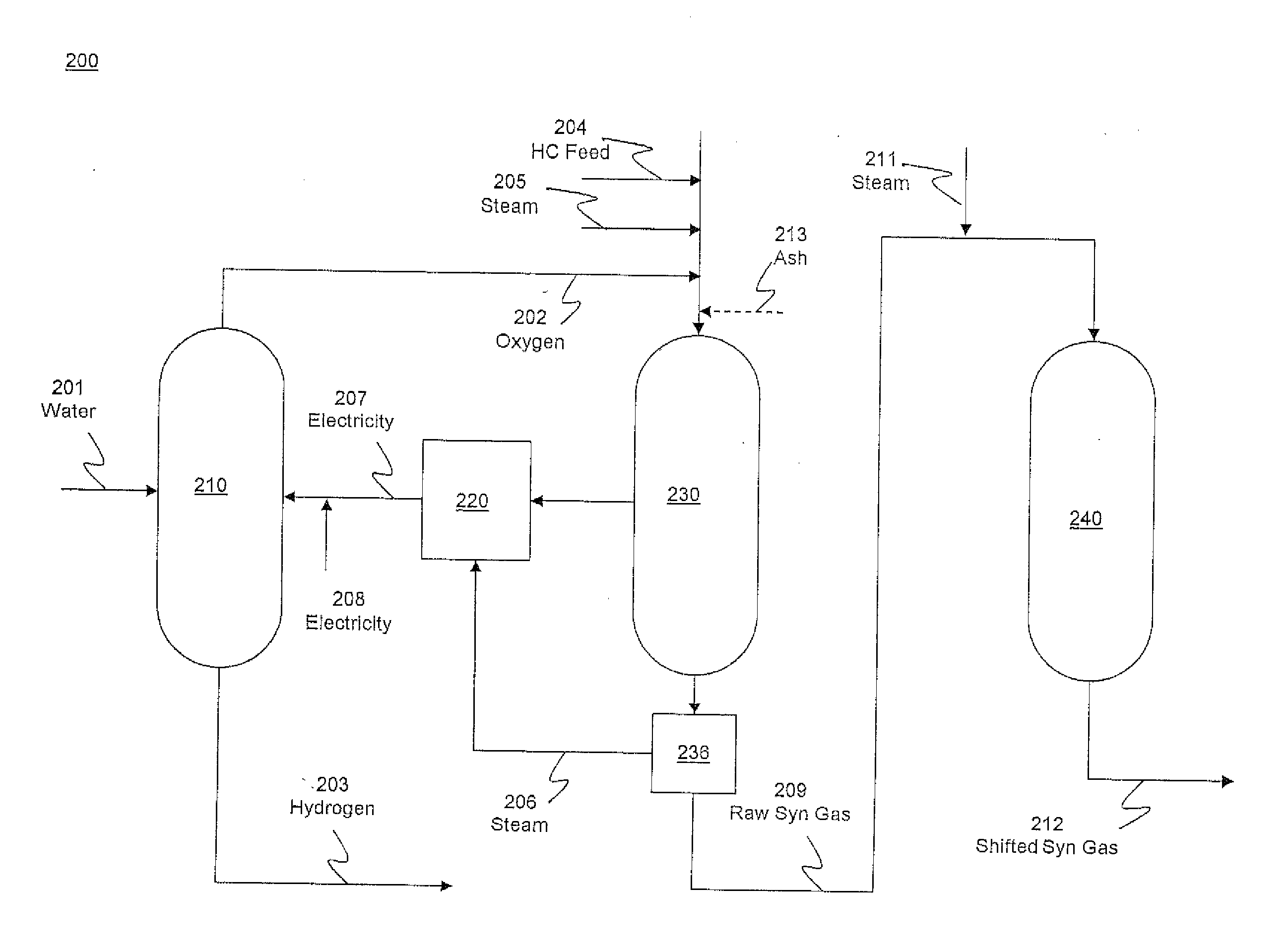
Hydrogen production from an integrated electrolysis cell and hydrocarbon gasification reactor
An integrated process for hydrogen gas production includes:a. operating a water electrolysis cell with an external source of electricity to produce oxygen and hydrogen;b. optionally operating an air separation unit to produce additional oxygen for the process;c. introducing a hydrocarbon feedstock into a membrane wall gasification reactor with an ash-forming material and steam, and oxygen from the electrolysis cell and, optionally, oxygen from the air separation unit to produce hot raw synthesis gas;d. passing the hot raw synthesis gas from the gasification reactor to a steam-generating heat exchanger to produce steam and a cooled raw synthesis gas;e. introducing the steam generated in the heat exchanger into a turbine to produce electricity to operate the electrolysis cell; andf. recovering the hydrogen gas from the water electrolysis cell and, optionally, subjecting the synthesis gas to a water-gas shift reaction to increase the hydrogen content and recovering the hydrogen.
Owner:SAUDI ARABIAN OIL CO
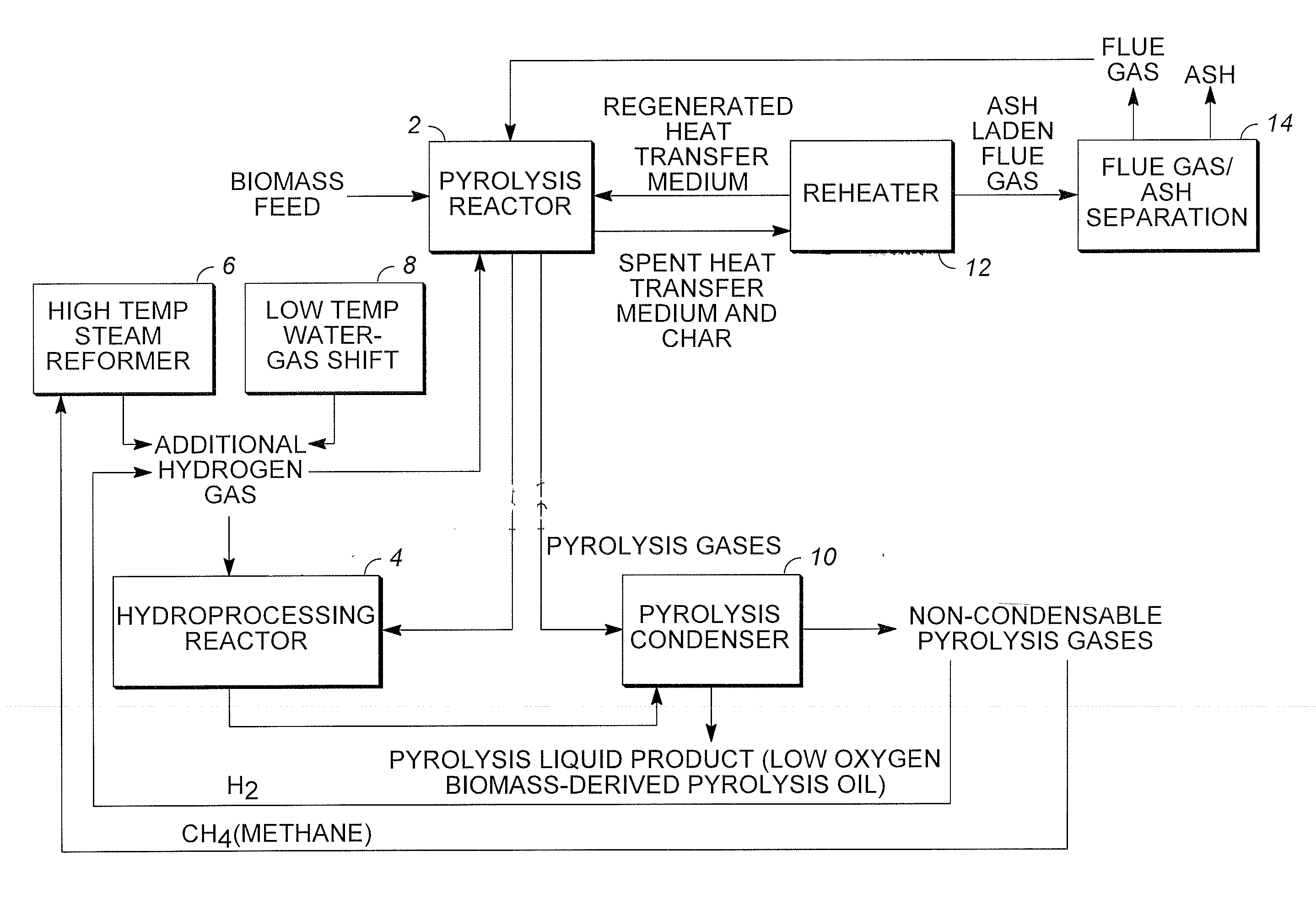
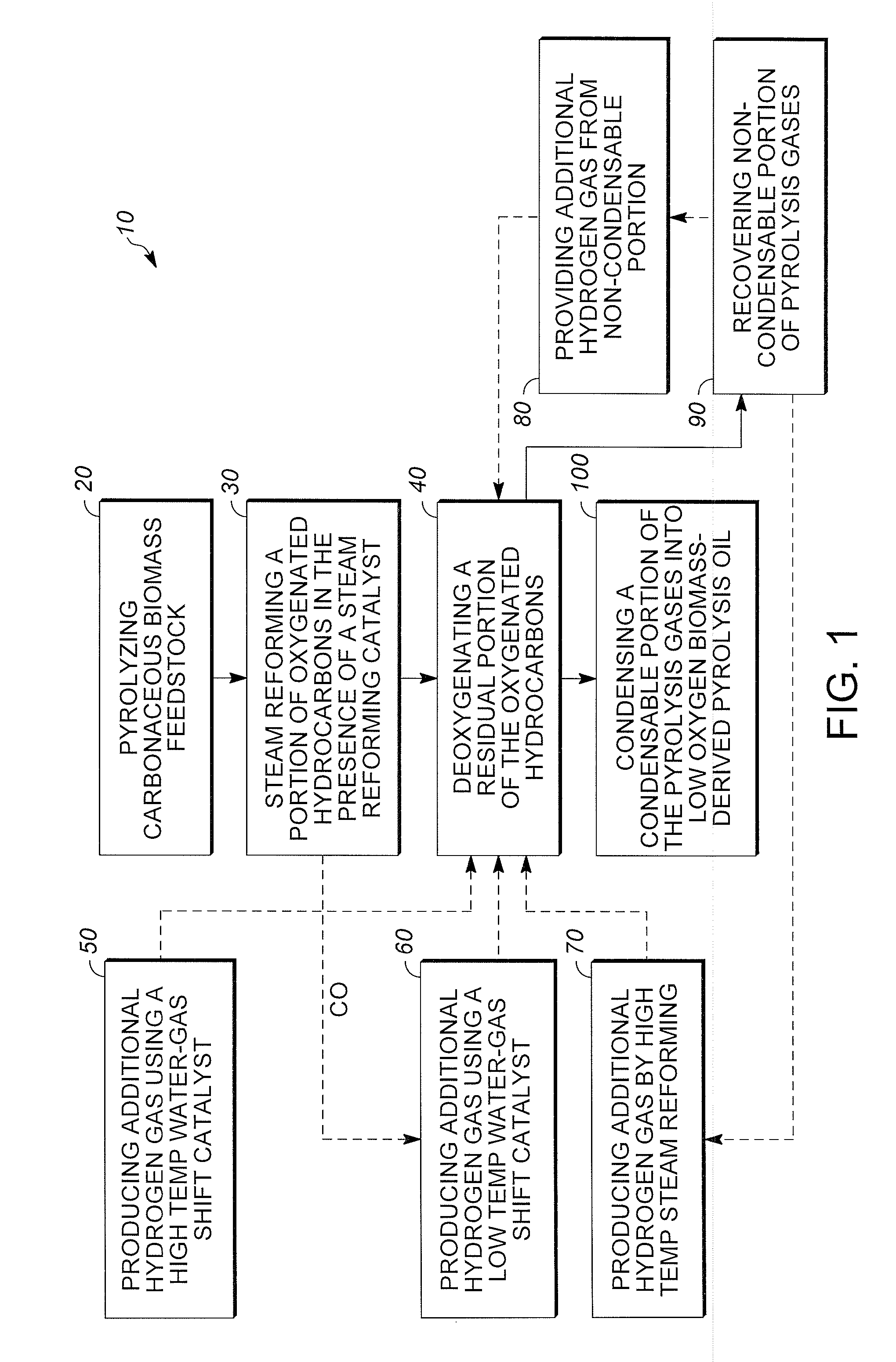
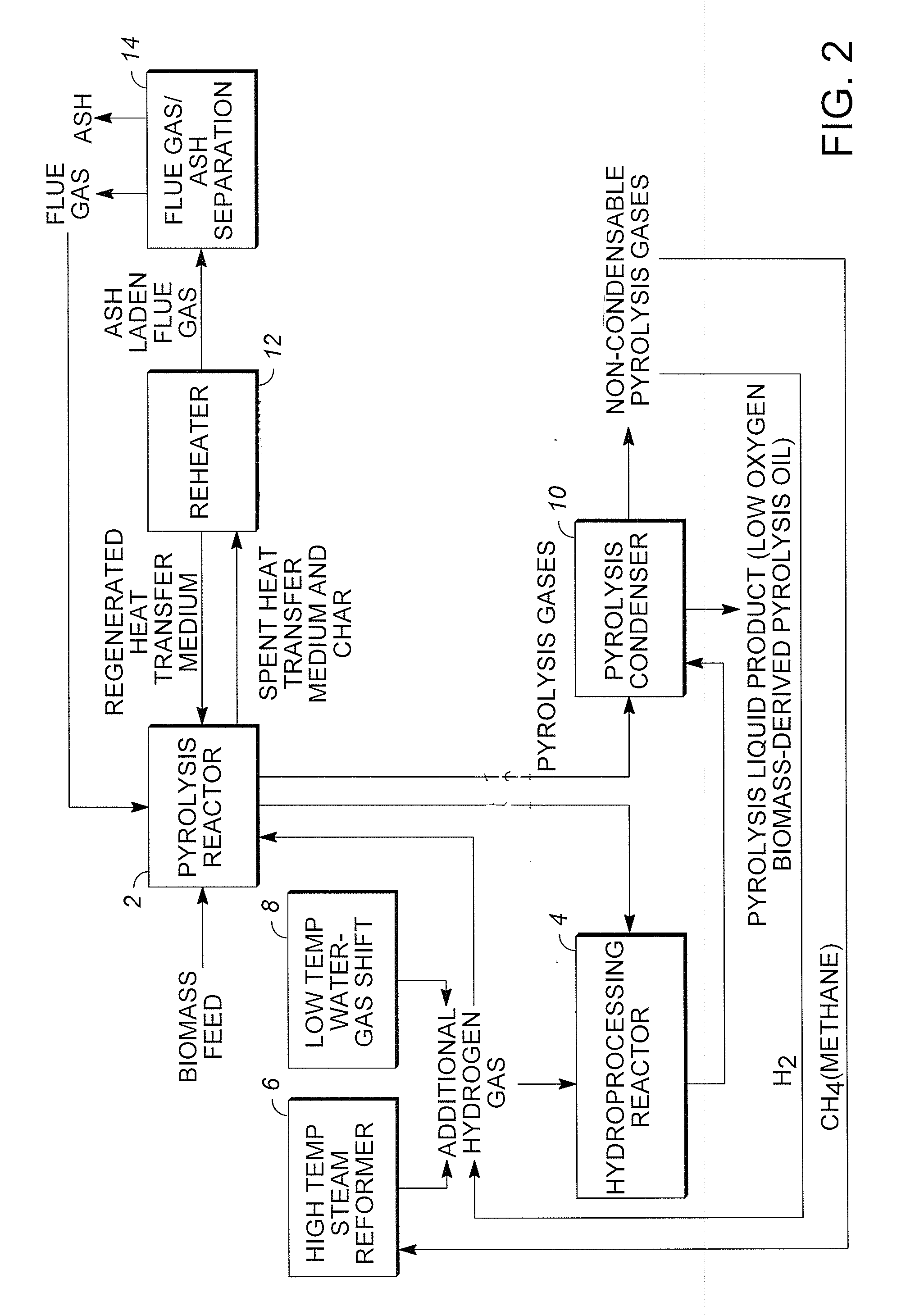
Low oxygen biomass-derived pyrolysis oils and methods for producing the same
InactiveUS20110201854A1Direct heating destructive distillationBiofuelsSteam reformingPartial oxidation
Methods are provided for producing low oxygen biomass-derived pyrolysis oil from carbonaceous biomass feedstock. The carbonaceous biomass feedstock is pyrolyzed in the presence of a steam reforming catalyst to produce char and pyrolysis gases. During pyrolysis, a portion of the oxygenated hydrocarbons in the pyrolysis gases is converted into hydrocarbons by steam reforming also yielding carbon oxides and hydrogen gas. The hydrogen gas at least partially deoxygenates a residual portion of the oxygenated hydrocarbons. Additional hydrogen gas may also be produced by water-gas shift reactions to deoxygenate the residual portion of the oxygenated hydrocarbons in the pyrolysis gases. Deoxygenation may occur in the presence of a hydroprocessing catalyst. A condensable portion of the pyrolysis gases is condensed to form low oxygen biomass-derived pyrolysis oil.
Owner:UOP LLC
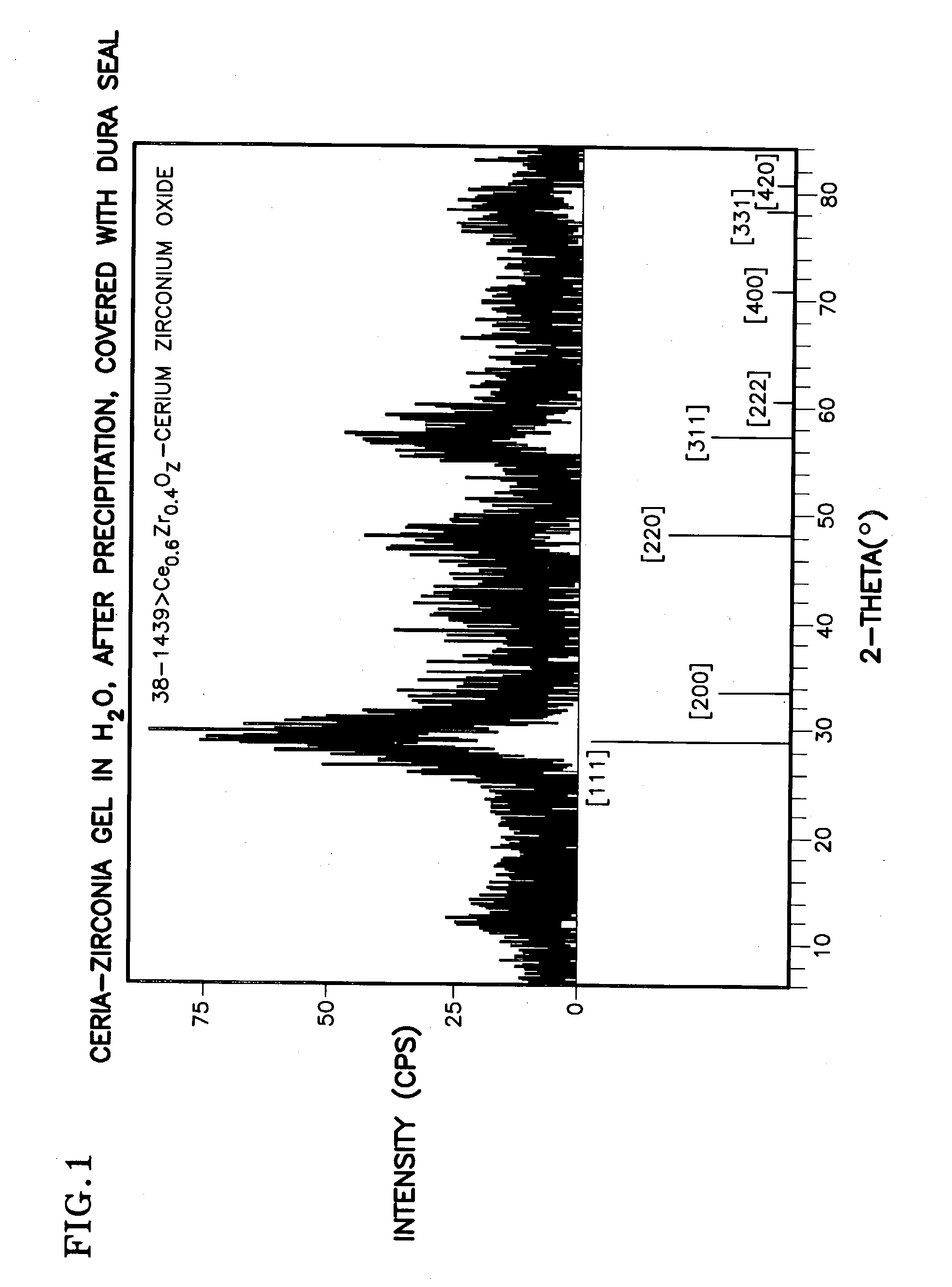
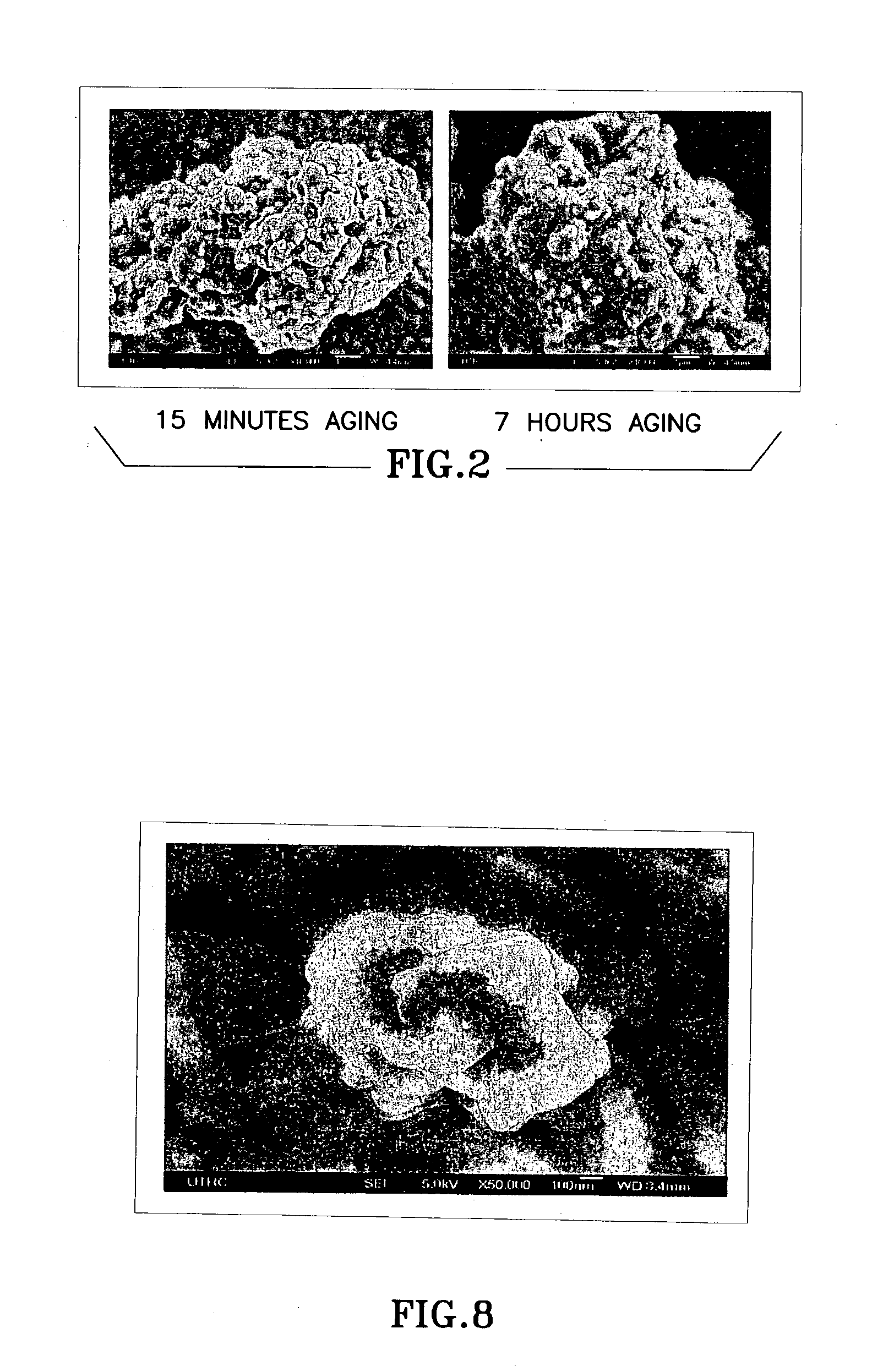
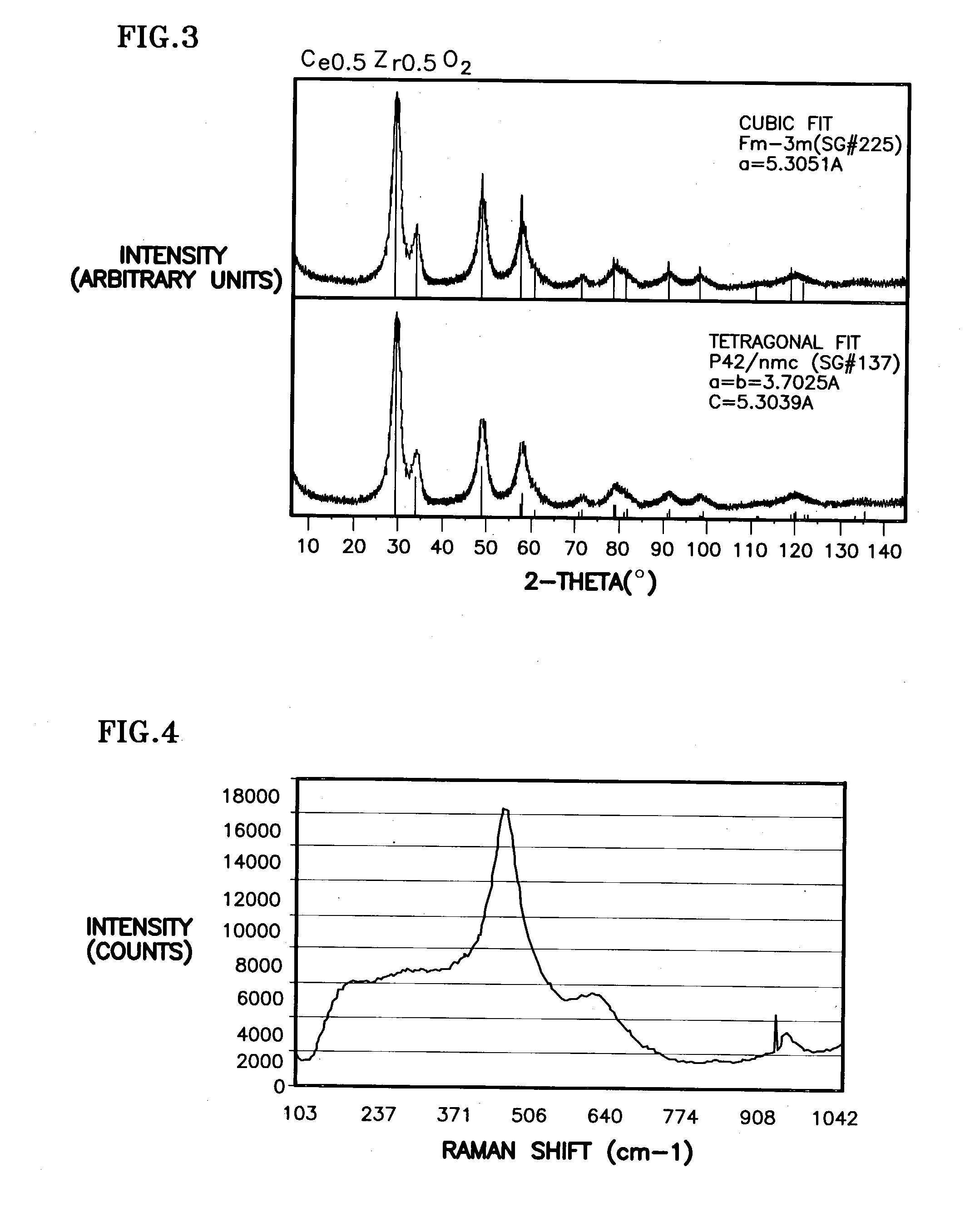
Ceria-based mixed-metal oxide structure, including method of making and use
InactiveUS20030235526A1Increased internal surface areaHigh catalytic activityRare earth metal oxides/hydroxidesMaterial nanotechnologyRheniumFuel cells
A homogeneous ceria-based mixed-metal oxide, useful as a catalyst support, a co-catalyst and / or a getter has a relatively large surface area per weight, typically exceeding 150 m<2> / g, a structure of nanocrystallites having diameters of less than 4 nm, and including pores larger than the nanocrystallites and having diameters in the range of 4 to about 9 nm. The ratio of pore volumes, VP, to skeletal structure volumes, VS, is typically less than about 2.5, and the surface area per unit volume of the oxide material is greater than 320 m<2> / cm<3>, for low internal mass transfer resistance and large effective surface area for reaction activity. The mixed metal oxide is ceria-based, includes Zr and or Hf, and is made by a novel co-precipitation process. A highly dispersed catalyst metal, typically a noble metal such as Pt, may be loaded on to the mixed metal oxide support from a catalyst metal-containing solution following a selected acid surface treatment of the oxide support. Appropriate ratioing of the Ce and other metal constituents of the oxide support contribute to it retaining in a cubic phase and enhancing catalytic performance. Rhenium is preferably further loaded on to the mixed-metal oxide support and passivated, to increase the activity of the catalyst. The metal-loaded mixed-metal oxide catalyst is applied particularly in water gas shift reactions as associated with fuel processing systems, as for fuel cells.
Owner:AUDI AG
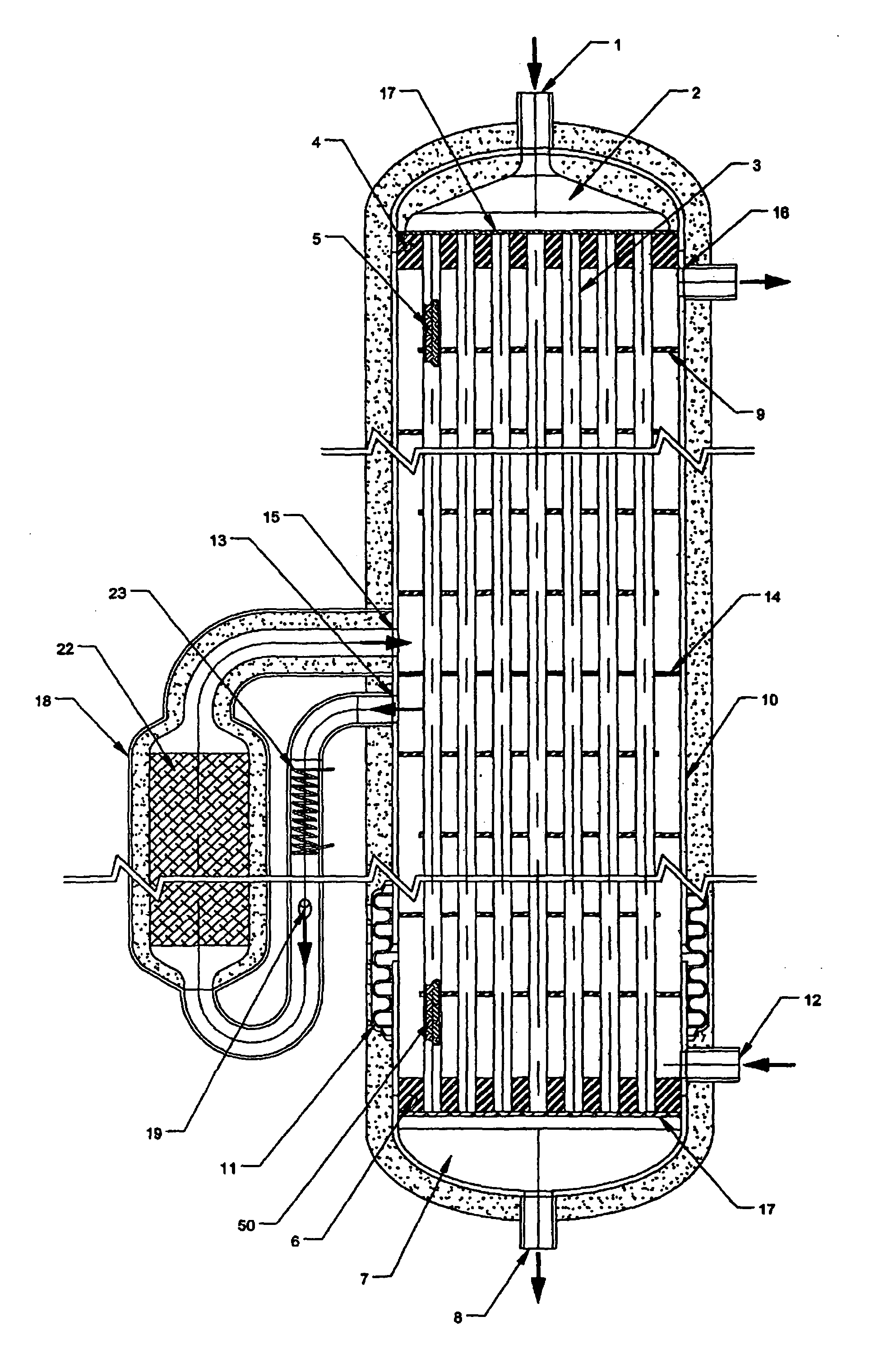
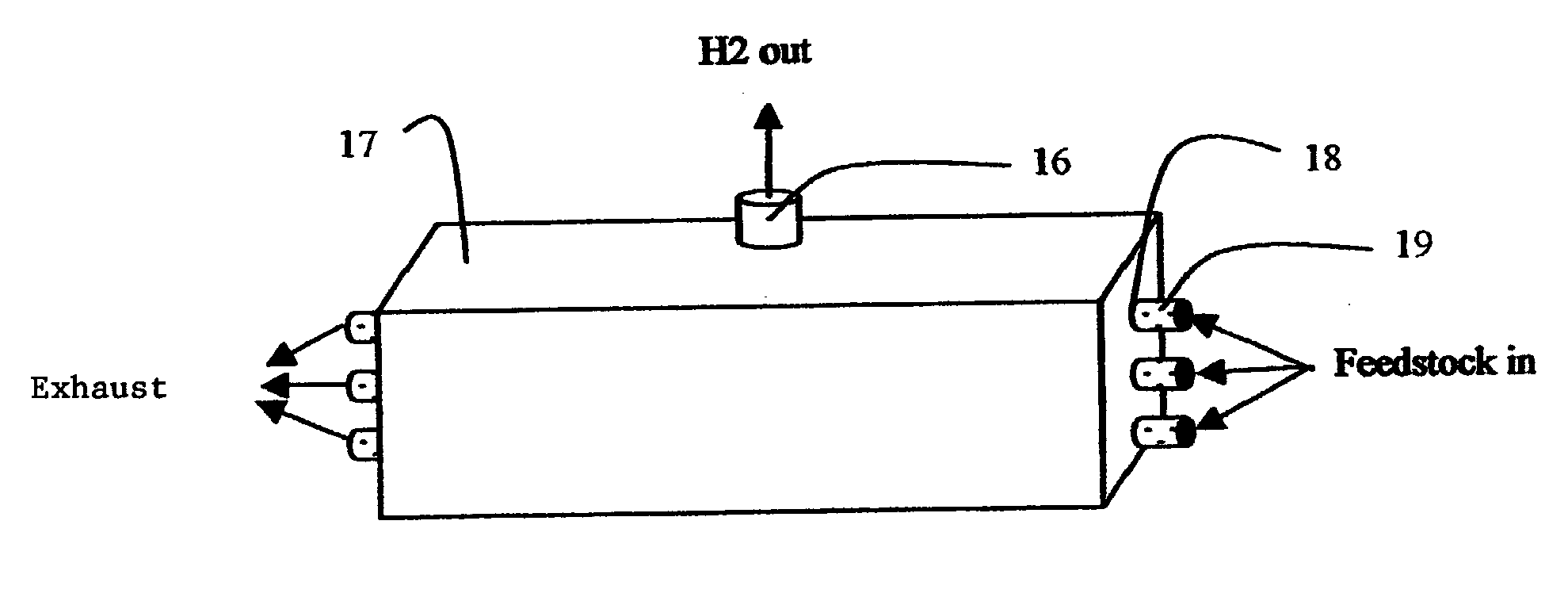
System for hydrogen generation through steam reforming of hydrocarbons and intergrated chemical reactor for hydrogen production from hydrocarbons
InactiveUS20050097819A1Avoid problemsCost efficientHydrogenHydrogen/synthetic gas productionSteam reformingEngineering
The present invention provides a reactor, which includes: a unitary shell assembly having an inlet and an outlet; a flow path extending within the shell assembly from the inlet to the outlet, the flow path having a steam reformer section with a first catalyst and a water gas shift reactor section with a second catalyst, the steam reformer section being located upstream of the water gas shift reactor section; a heating section within the shell assembly and configured to heat the steam reformer section; and a cooling section within the shell assembly and configured to cool the water gas shift reactor section. The present invention also provides a simplified hydrogen production system, which includes the catalytic steam reforming and subsequent high temperature water gas shift of low-sulfur (<100 ppm by mass) hydrocarbon fuels followed by hydrogen purification through the pressure swing adsorption (PSA). The integrated reactor offers significant advantages such as lower heat loss, lower parts count, lower thermal mass, and greater safety than the many separate components employed in conventional and is especially well-suited to applications where less than 15,000 standard cubic feet per hour of hydrogen are required. The improved system also may be started, operated and shut down more simply and quickly than what is currently possible in conventional systems. The improved system preferably employs active temperature control for added safety of operation. The hydrogen product is of high purity, and the system may be optionally operated with a feedback control loop for added purity.
Owner:H2GEN INNOVATIONS INC
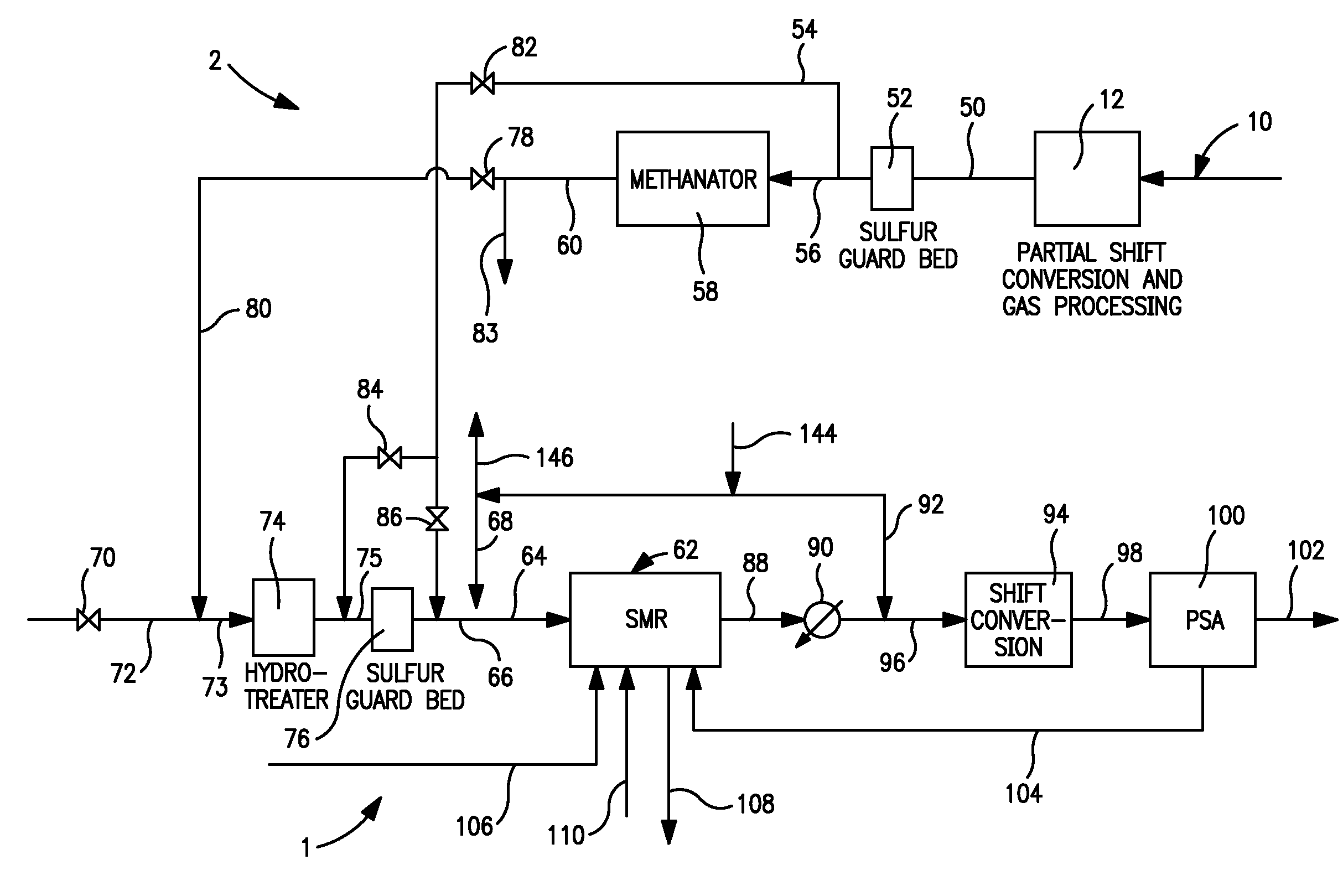
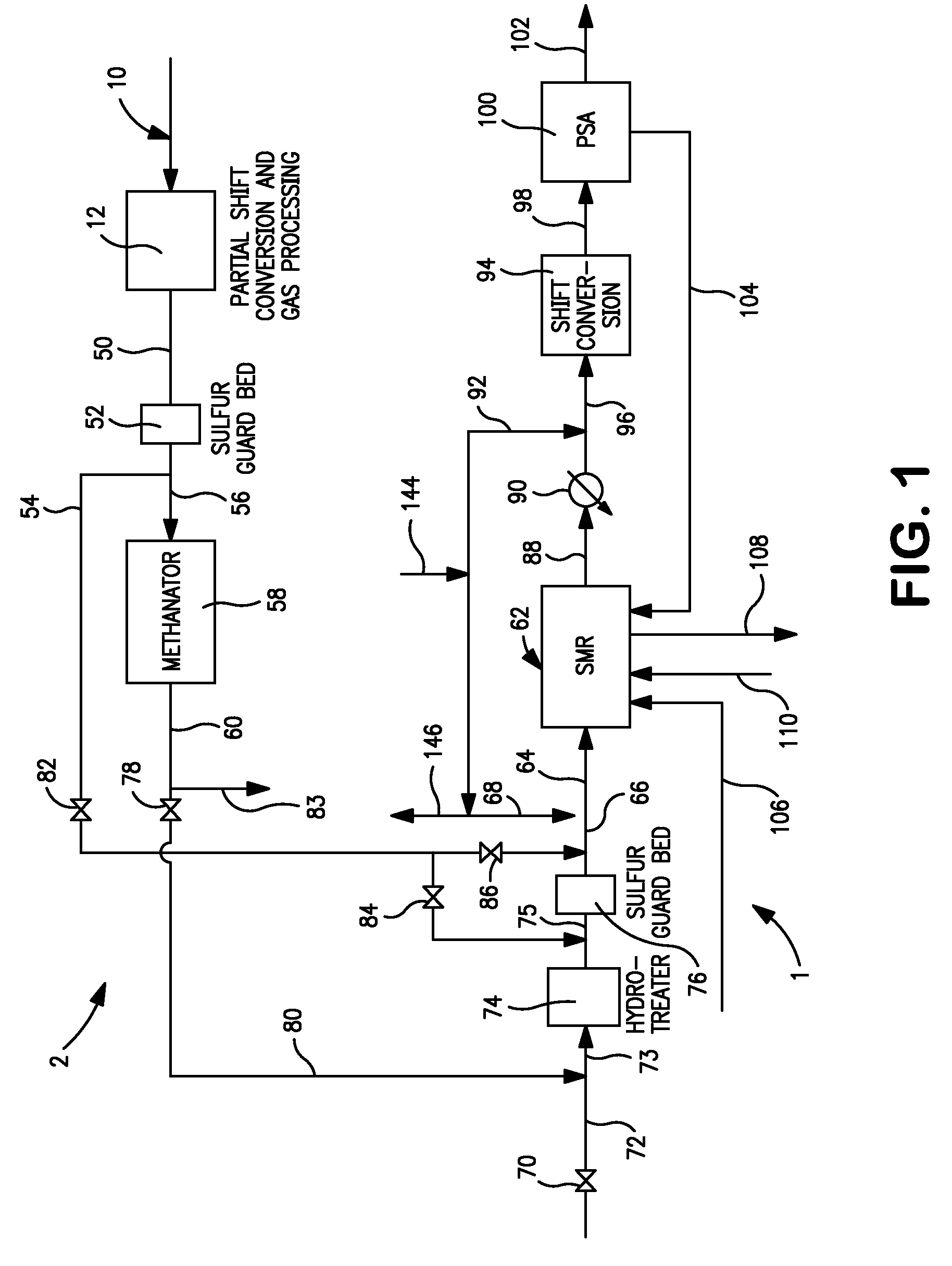
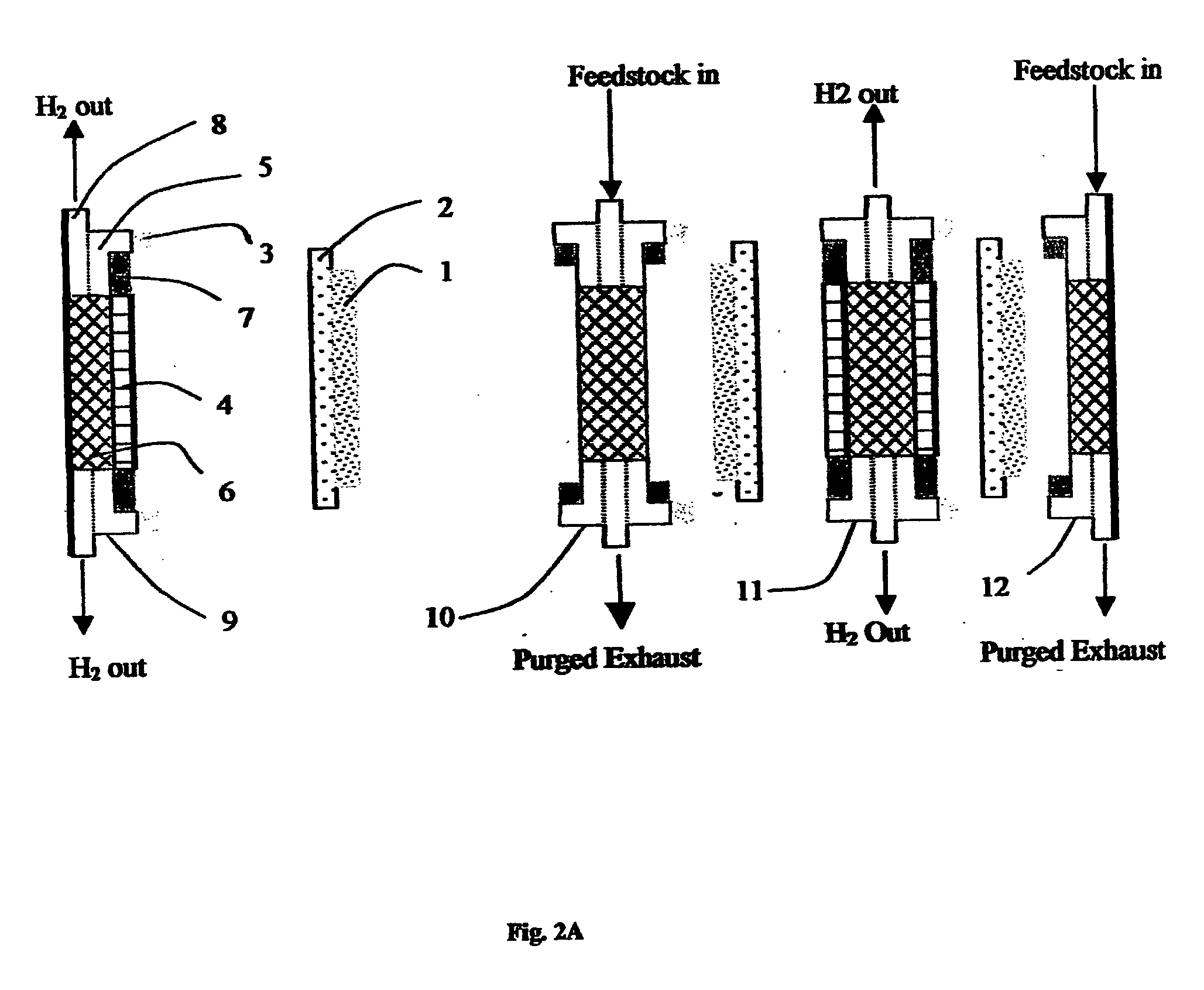
Process and apparatus for production of hydrogen using the water gas shift reaction
InactiveUS20080128655A1Lower carbon dioxide levelsCatalytic gas-gas reactionHydrogenForming gasWater-gas shift reaction
A process and a reactor vessel for production of hydrogen via the water gas shift reaction at CO / CO2 ratios above 1.9, and steam to gas rations below 0.5, are disclosed. The process includes first reacting a feed gas mixture of carbon monoxide and steam in the presence of a precious metal catalyst on a structural support, yielding a resultant gas, and then reacting the resultant gas in the presence of a non-precious metal catalyst on a support medium. The reactor vessel includes a chamber having an inlet duct and an outlet. A structural support having the precious metal catalyst is positioned upstream of the non-precious metal catalyst positioned within the chamber. The structural support may be positioned within the inlet duct or within the chamber. The support medium may be a granular medium or a structural support.
Owner:AIR PROD & CHEM INC
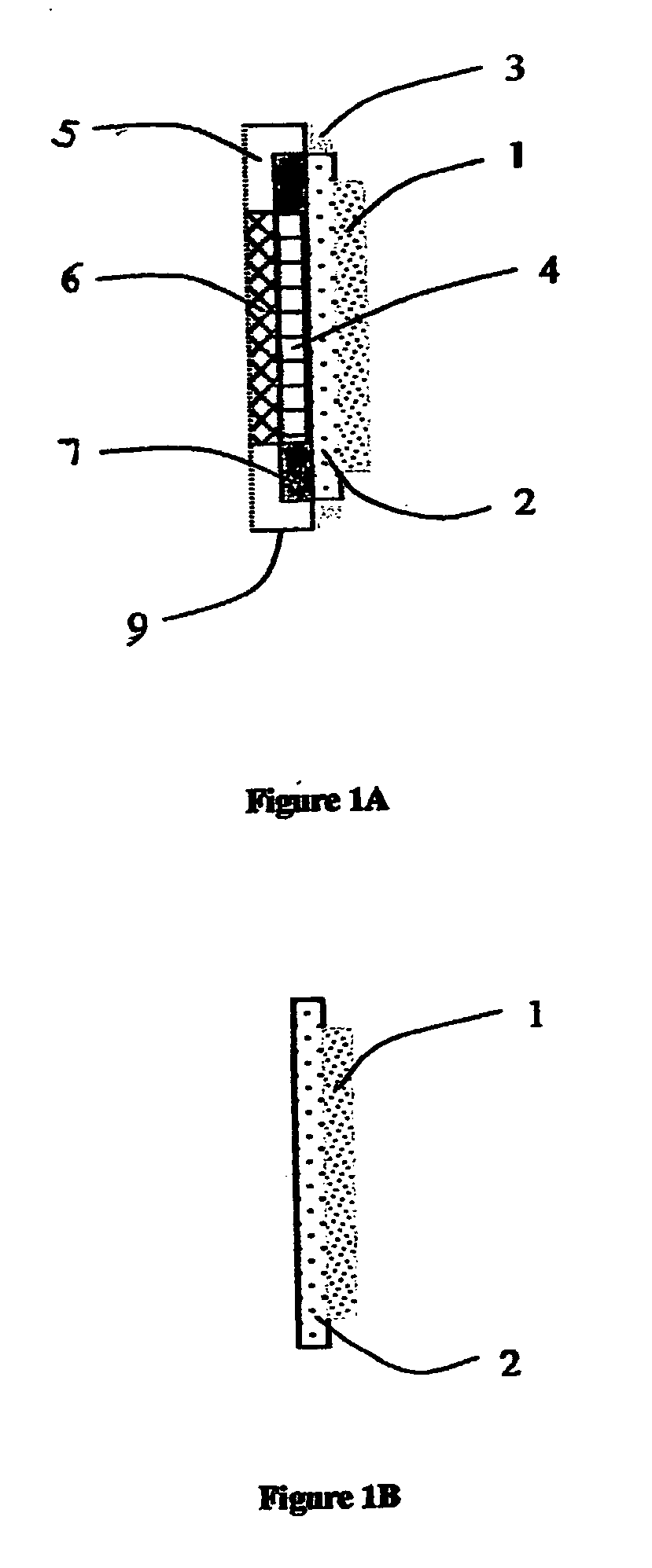
Single stage membrane reactor for high purity hydrogen production
InactiveUS20070157517A1Improve responseEasy to operateCatalytic gas-gas reactionMembranesSteam reformingMembrane reactor
Hydrogen generating method and apparatus. The apparatus comprises a mixed phase protonic-electron conducting cermet membrane (2), wherein said membrane is coated with porous metal or composite catalyst (1) effective to decompose hydro-carbon and water reactants into a hydrogen-rich syngas at elevated temperature and pressure. The hydrogen ions are continuously withdrawn in situ by diffusing them through the mixed phase conducting cermet membrane (2) to the second membrane side where the hydrogen ions can be reconstituted into molecular hydrogen with a supply of electrons. The method favorably shifts the equilibrium of steam reforming and water gas shift reaction by use a single stage, high efficiency and high purity membrane reactor.
Owner:TSAY DAVID +2
Pumped carbon mining methane production process
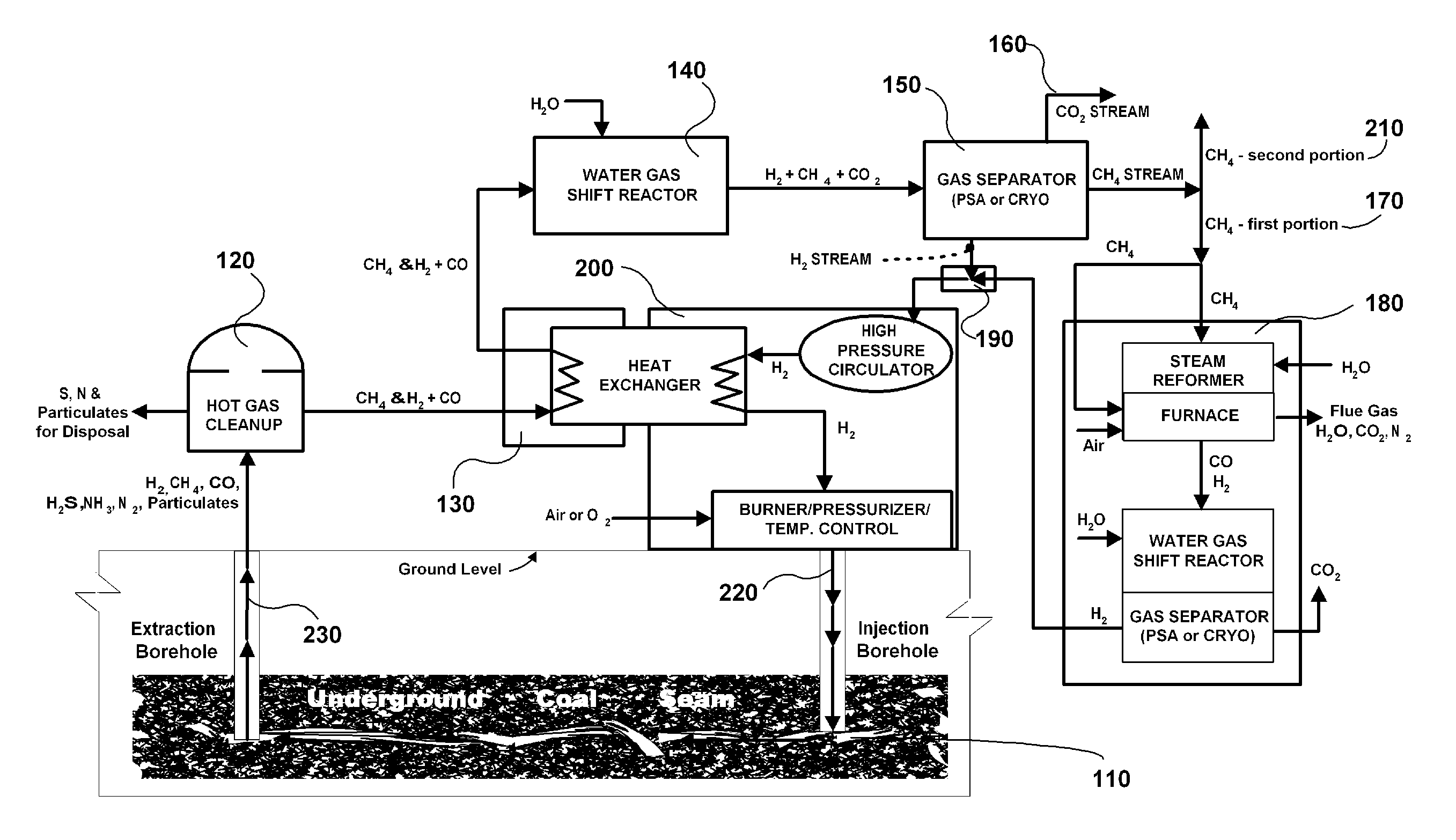
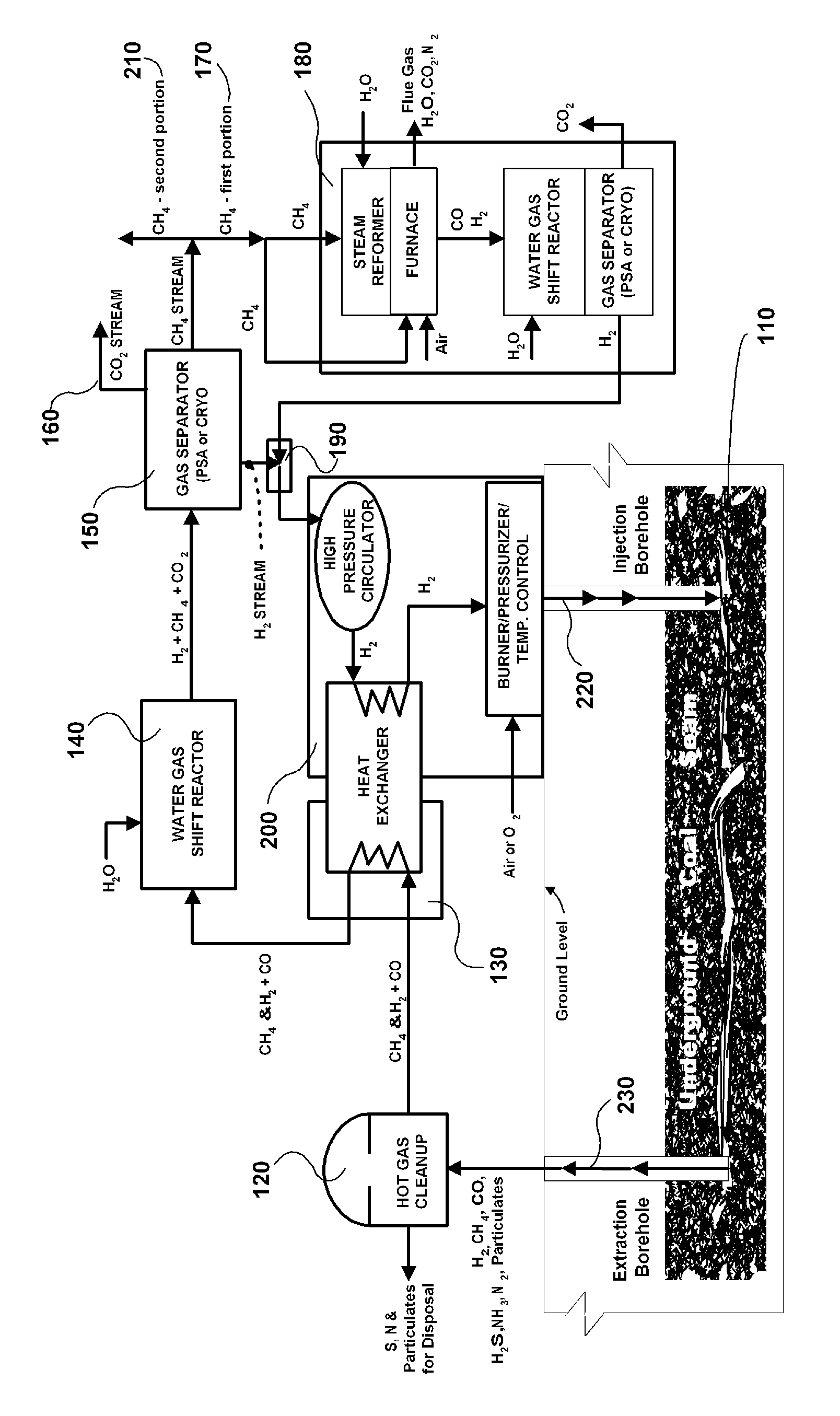
The invention is a continuous process for producing methane from an underground coal bed or an above ground carbon-containing resource using hydrogen as a recycling working fluid. For an underground coal seam, the process includes injecting (220) hydrogen into the coal seam to form a reaction effluent of methane, hydrogen and carbon monoxide; extracting the reaction effluent (230) for processing above ground; cleaning the reaction effluent (120); cooling the cleaned reaction effluent (130) and processing it through a water gas shift reactor (140); separating (150) hydrogen, methane, and carbon dioxide into separate streams; producing the carbon dioxide stream (160) as a product gas; processing a first portion (170) of the methane stream in a steam reformer, water gas shift reactor and gas separator (180) to produce segregated flows of hydrogen and carbon dioxide, combining the segregated hydrogen flow with the separated hydrogen stream (190); heating and repressurizing (200) the combined hydrogen stream to the temperature and pressure of the hydrogen in the first step; producing a second portion (210) of said methane stream as a product gas; and injecting (220) the combined hydrogen stream into the underground coal bed to continue the process.
Owner:HCE
Catalysts for hydrogen production
ActiveUS7824656B2Improve thermal stabilityIncrease temperatureHydrogenMolecular sieve catalystsPtru catalystWater-gas shift reaction
The present invention relates to catalysts for the production of hydrogen using the water gas shift reaction and the carbon dioxide reforming of hydrocarbon-containing fuels. The catalysts nickel and / or copper on a ceria / zirconia support, where the support is prepared using a surfactant templating method. The invention also includes processes for producing hydrogen, reactors and hydrogen production systems utilizing these catalysts.
Owner:UNIVERSITY OF REGINA
Method for conversion of atmospheric carbon dioxide into useful materials
A method for converting carbon dioxide in a gaseous environment, including air into useful materials by use of renewable energy sources which comprises:(1) extracting carbon dioxide from a gaseous source using a sorbent such as sodium hydroxide, calcium hydroxide, or potassium hydroxide;(2) utilizing wind power, solar power, or other renewable energy sources to regenerate said sorbent by membrane cell electrolysis or other similar method, simultaneously producing hydrogen (H2) gas;(3) releasing carbon dioxide from said sorbent; and(4) utilizing the Fischer Tropsch process, Mobil process, ICI process, or related or similar processes to convert carbon oxides to a hydrocarbon concomittantly with or after effecting the reverse water-gas shift reaction to convert said CO2 and H2 gas into carbon monoxide for reaction in said Fischer Tropsch process, Mobil process or ICI process.
Owner:KAPLAN THOMAS PROGER
Catalyst and sorbent material for the production of hydrogen
A catalyst and sorbent is disclosed which comprises pellets with an absorbent core and a protective shell with a catalyst in the shell. Such material is especially well suited for steam reforming of hydrocarbons to produce hydrogen since a reforming catalyst can be incorporated in the shell and a sorbent for the by-product carbon dioxide can be used for the core. It is also well suited for producing hydrogen from carbon monoxide by means of the water gas shift reaction. The shell can be made sufficiently strong and durable for moving bed applications as well as fixed bed applications.
Owner:IOWA STATE UNIV RES FOUND
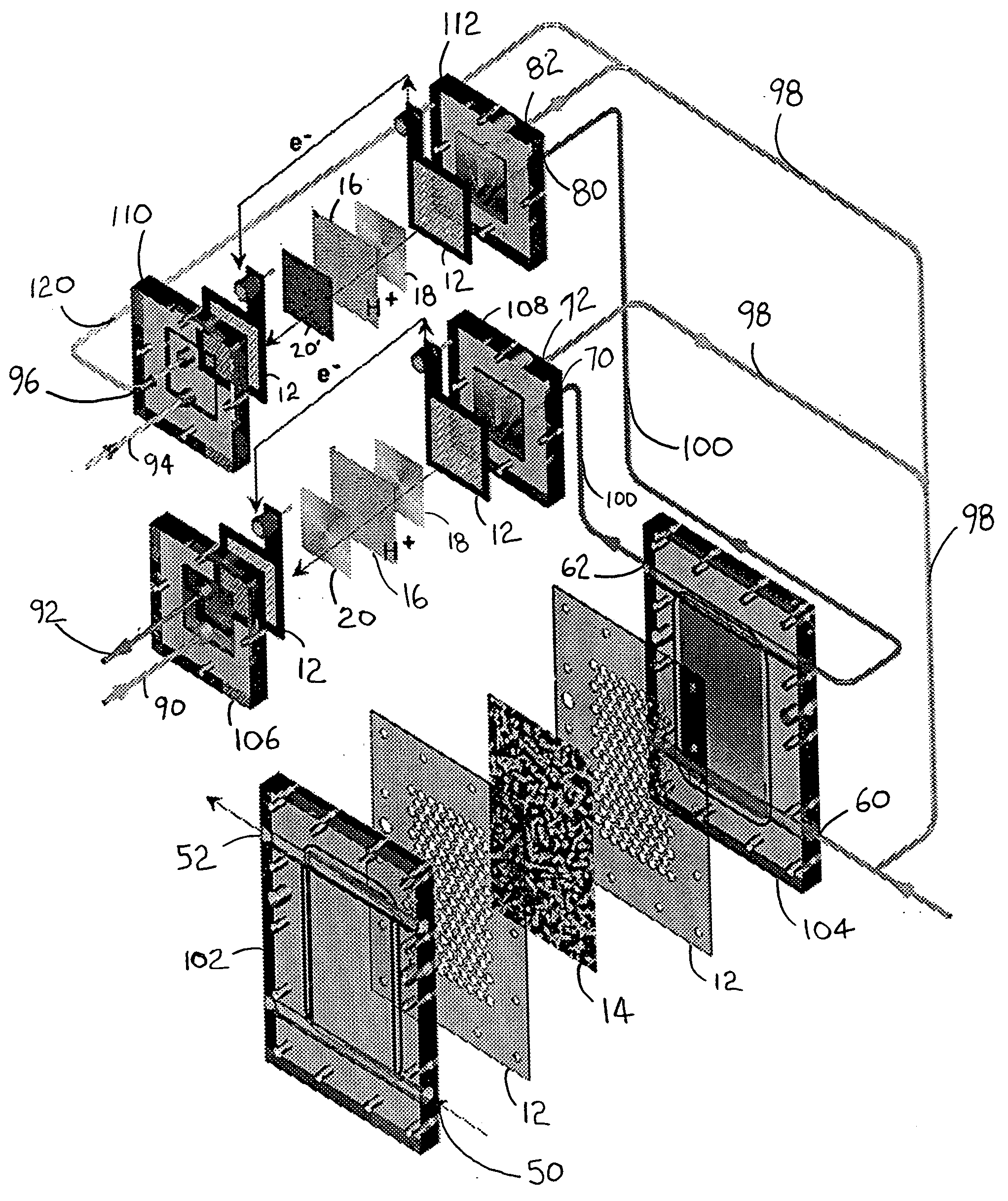
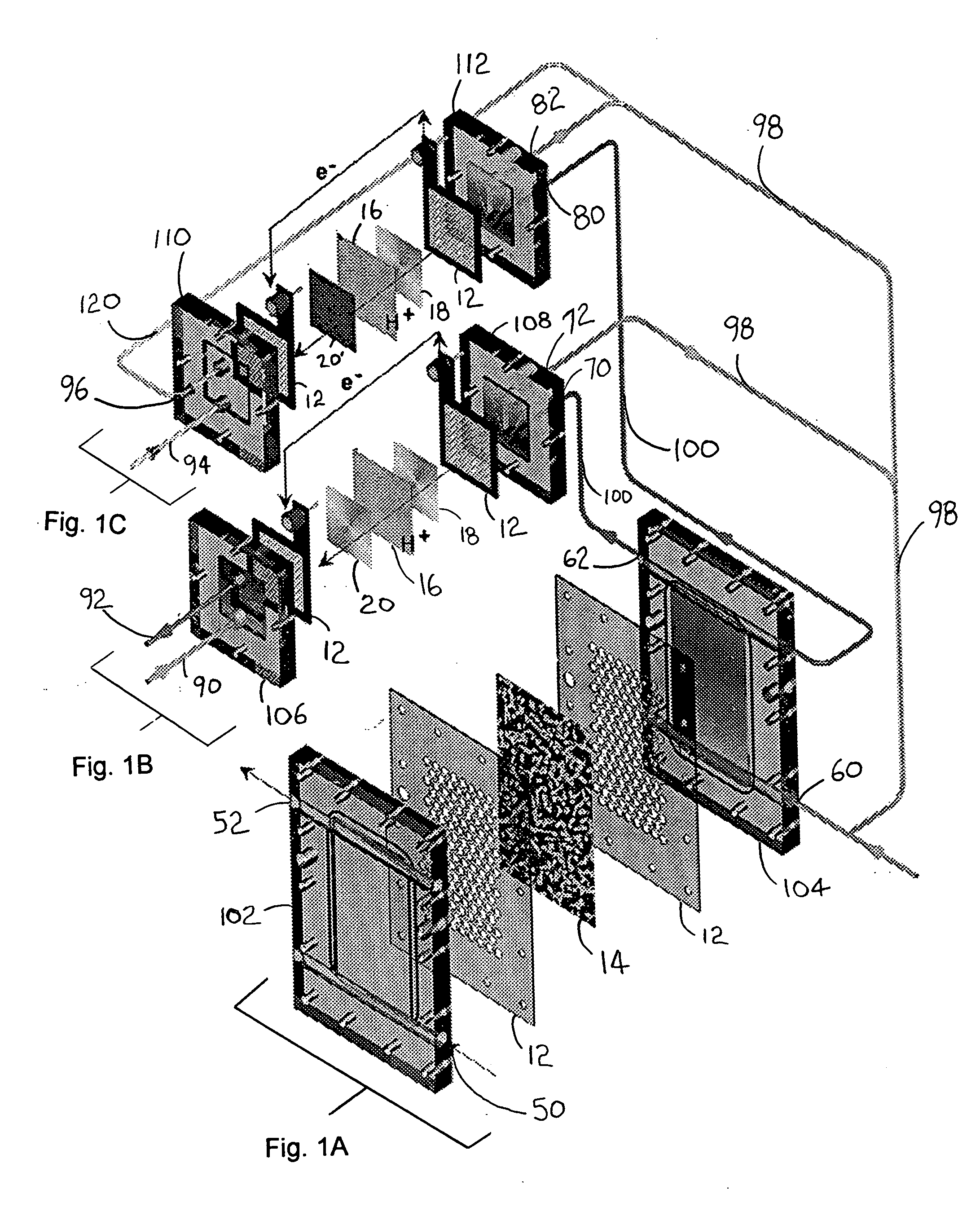
Carbon monoxide adsorption for carbon monoxide clean-up in a fuel cell system
InactiveUS6964692B2Eliminate useReduce carbon monoxide contentThermal non-catalytic crackingCombination devicesSorbentHydrogen fuel cell
An apparatus removes carbon monoxide (CO) from a hydrogen-rich gas stream in a hydrogen fuel cell system. CO fouls costly catalytic particles in the membrane electrode assemblies of proton exchange membrane (PEM) fuel cells. A vessel houses a carbon monoxide adsorbent. The vessel may be a rotating pressure swing adsorber. A water gas shift reactor is upstream of the rotating pressure swing adsorber. The water gas shift reactor may include a second adsorbent adapted to adsorb carbon monoxide at low temperatures and to desorb carbon monoxide at high temperatures. The apparatus advantageously eliminates the use of a preferential oxidation (PROX) reactor, by providing an apparatus which incorporates CO adsorption in the place of the PROX reactor. This cleans up carbon monoxide without hydrogen consumption and the concomitant, undesirable excess low grade heat generation. The present invention reduces start-up duration, and improves overall fuel processor efficiency during normal operation.
Owner:GM GLOBAL TECH OPERATIONS LLC
Monoatomic dispersed noble metal catalyst and application thereof
ActiveCN107649124AHave substantive characteristicsEnhance intrinsic reactivityHydrogenCatalyst activation/preparationIridiumMethanation
The invention relates to a monoatomic dispersed platinum group metal based catalyst with a higher loading capacity and an application of the catalyst. Specifically, the invention relates to a bicomponent catalyst of titanium oxide and one selected from metals rhodium (Rh), ruthenium (Ru), platinum (Pt), iridium (Ir) and palladium (Pd) as well as preparation and an application of the catalyst. Themetal is highly dispersed in the form of a single atom on the carrier titanium oxide, and the metal content is up to 0.4-2%. The catalyst provided by the invention is suitable for the CO water gas shift reaction, and can improve the CO conversion rate and inhibit a methanation side reaction; and meanwhile, the catalyst also has very good low-temperature activity and use stability on catalytic decomposition of the liquid single-component propellant level anhydrous hydrazine used in a satellite attitude control engine in the aerospace industry.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of maintaining a fuel Wobbe index in an IGCC installation
InactiveUS20070082306A1Low indexFuel supply regulationGaseous fuel feeder/distributionWater-gas shift reactionProcess engineering
A method of reducing a modified Wobbe index of a fuel stream fed to diffusion combustors of a gas turbine that is used in connection with an IGCC installation in which a nitrogen stream is fed into the head ends of the combustor of NOx control and the modified Wobbe index of the fuel stream has been increased in an amount that is greater than at least about 10 percent of a design Wobbe index for the fuel to be fed to the gas turbine. The reason for the increase is the conversion of the carbon monoxide within the fuel stream to hydrogen and carbon dioxide by a water gas shift reaction and subsequent removal of the carbon dioxide. The nitrogen stream is maintained at the same level both with and without conversion and subsequent removal of carbon atoms. The nitrogen stream is divided to subsidiary streams that are respectively fed into the head end of the combustors and that are mixed with a fuel to lower the modified Wobbe index to acceptable levels for the gas turbine.
Owner:PRAXAIR TECH INC
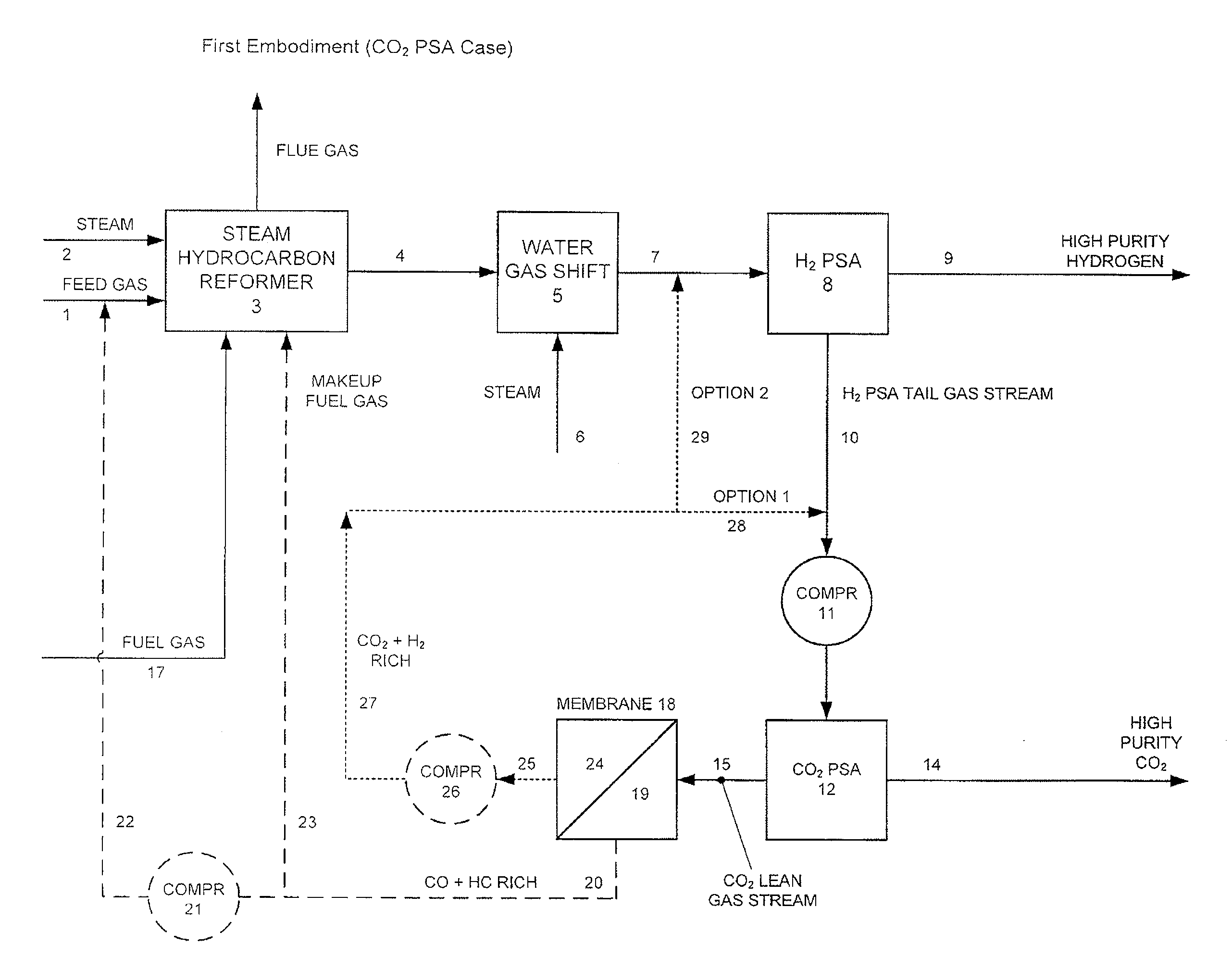
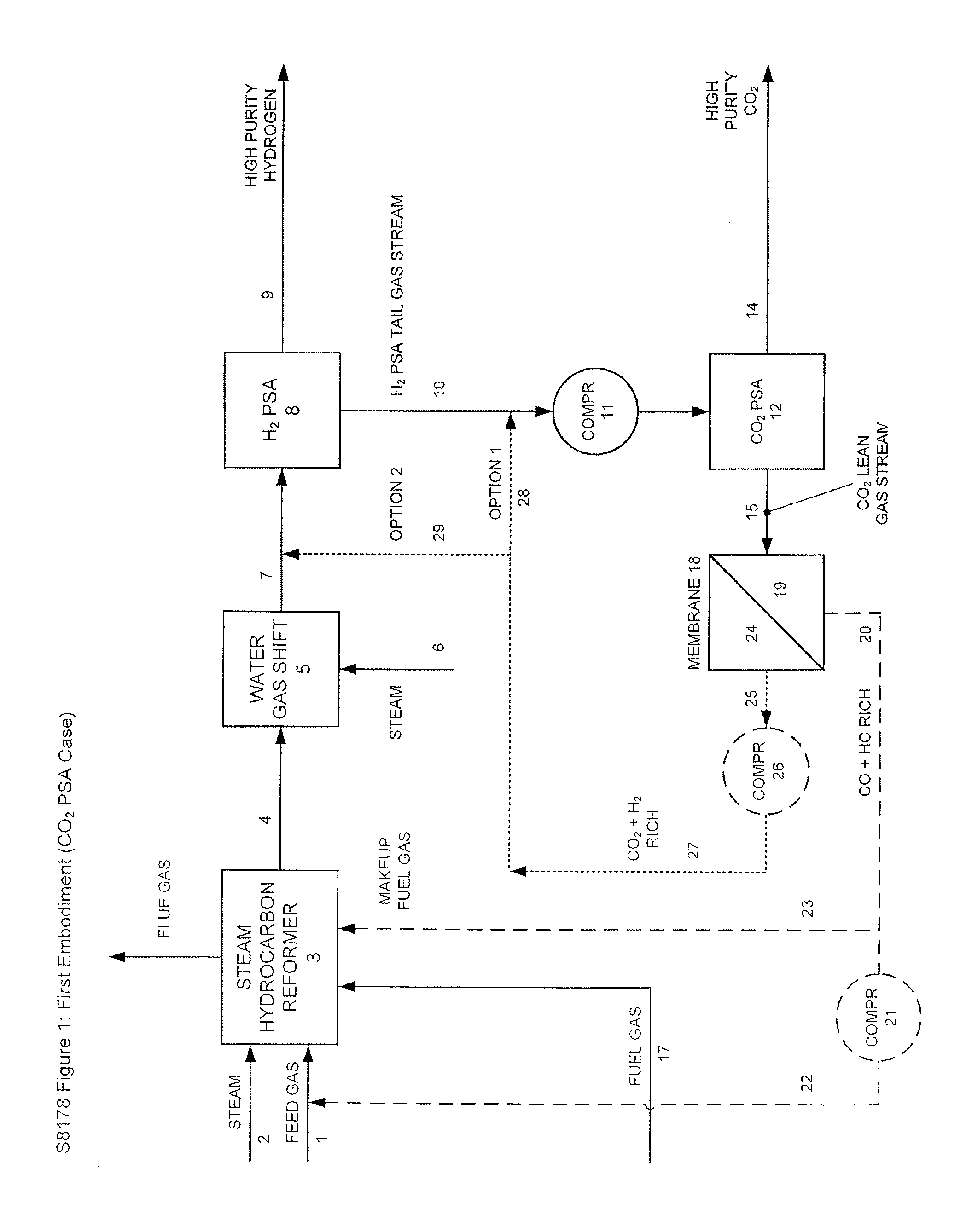
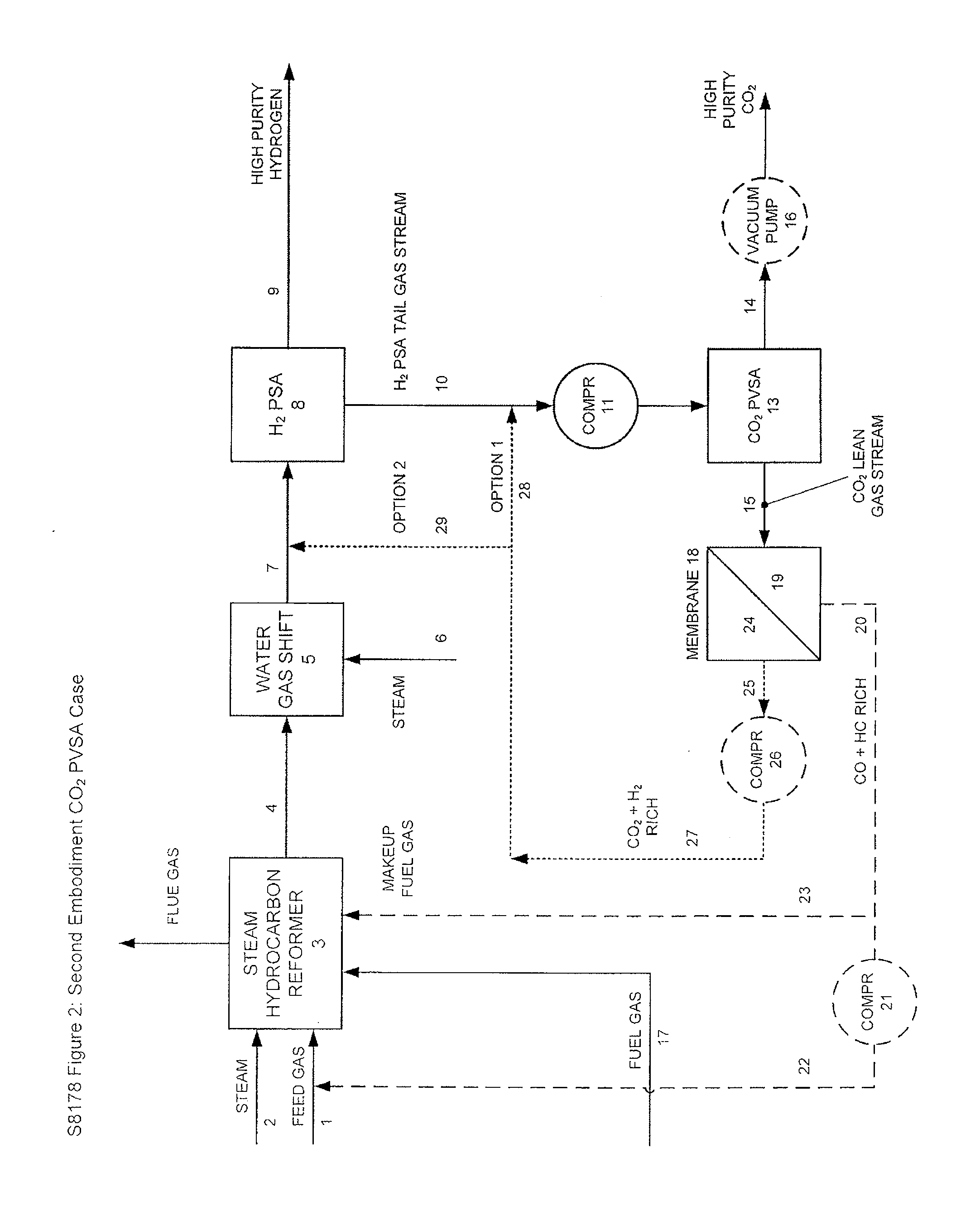
Processes For The Recovery Of High Purity Hydrogen And High Purity Carbon Dioxide
The present invention provides for various processes for recovering high purity gaseous hydrogen and high purity gaseous carbon dioxide from the gas stream produced using steam hydrocarbon reforming, especially steam methane reforming, utilizing a H2 pressure swing adsorption unit in combination with either a CO2 pressure swing adsorption unit in combination with a membrane separation unit or a CO2 pressure vacuum swing adsorption unit in combination with a membrane separation unit. The present invention further relates to a process for optimizing the recovery of carbon dioxide from waste gas streams produced during the hydrogen purification step of a steam hydrocarbon reforming / water gas shift reactor / H2 pressure swing adsorption unit utilizing either a CO2 pressure swing adsorption unit in combination with a membrane separation unit or a CO2 pressure vacuum swing adsorption unit in combination with a membrane separation unit. The present invention even further relates to the apparatus necessary to carry out the various processes of the present invention.
Owner:AIR LIQUIDE IND US LP +1
Process to prepare a sweet crude
ActiveUS20080142408A1Hydrocarbon distillationCombustible gas catalytic treatmentOil sandsRemoval Units
A process to prepare a sweet crude from an ash containing and heavy fraction of a tar sand oil by:(a) supplying an atmospheric distillation bottoms of a tar sands originated feed to a vacuum distillation to obtain a vacuum gas oil and a vacuum bottoms,(b) contacting the vacuum gas oil with hydrogen in the presence of a suitable hydrocracking catalyst to obtain a sweet synthetic crude(c) separating the vacuum bottoms obtained in step (a) into an asphalt fraction comprising between 0.1 and 4 wt % ash and a de-asphalted oil,(d) feeding said asphalt fraction to a burner of a gasification reactor where the asphalt fraction is partially oxidized in the presence of an oxidizer gas in a burner to obtain a mixture of hydrogen and carbon monoxide,(e) performing a water gas shift reaction on the mixture of hydrogen and carbon monoxide,(f) separating hydrogen sulphide and carbon dioxide from the shifted gas in an acid removal unit thereby obtaining crude hydrogen,(g) purifying the crude hydrogen to obtain pure hydrogen and(h) using part of the pure hydrogen in step (b), wherein in step (d) the asphalt fraction is provided to the burner in a liquid state and wherein in case separation step (c) fails to provide sufficient feed for step (d), step (d) is performed by feeding the vacuum bottoms of step (a) to the burner in a liquid state.
Owner:AIR PROD & CHEM INC
Carbon dioxide production method
A method of producing a carbon dioxide product stream from a synthesis gas stream formed within a hydrogen plant having a synthesis gas reactor, a water-gas shift reactor, located downstream of the synthesis gas reactor to form the synthesis gas stream and a hydrogen pressure swing adsorption unit to produce a hydrogen product recovered from the synthesis gas stream. In accordance with the method the carbon dioxide from the synthesis gas stream by separating the carbon dioxide from the synthesis gas stream in a vacuum pressure swing adsorption system, thereby to produce a hydrogen-rich synthesis gas stream and a crude carbon dioxide stream and then purifying the crude carbon dioxide stream by a sub-ambient temperature distillation process thereby to produce the carbon dioxide product. A hydrogen synthesis gas feed stream to the hydrogen pressure swing adsorption unit is formed at least in part from the hydrogen rich stream.
Owner:PRAXAIR TECH INC
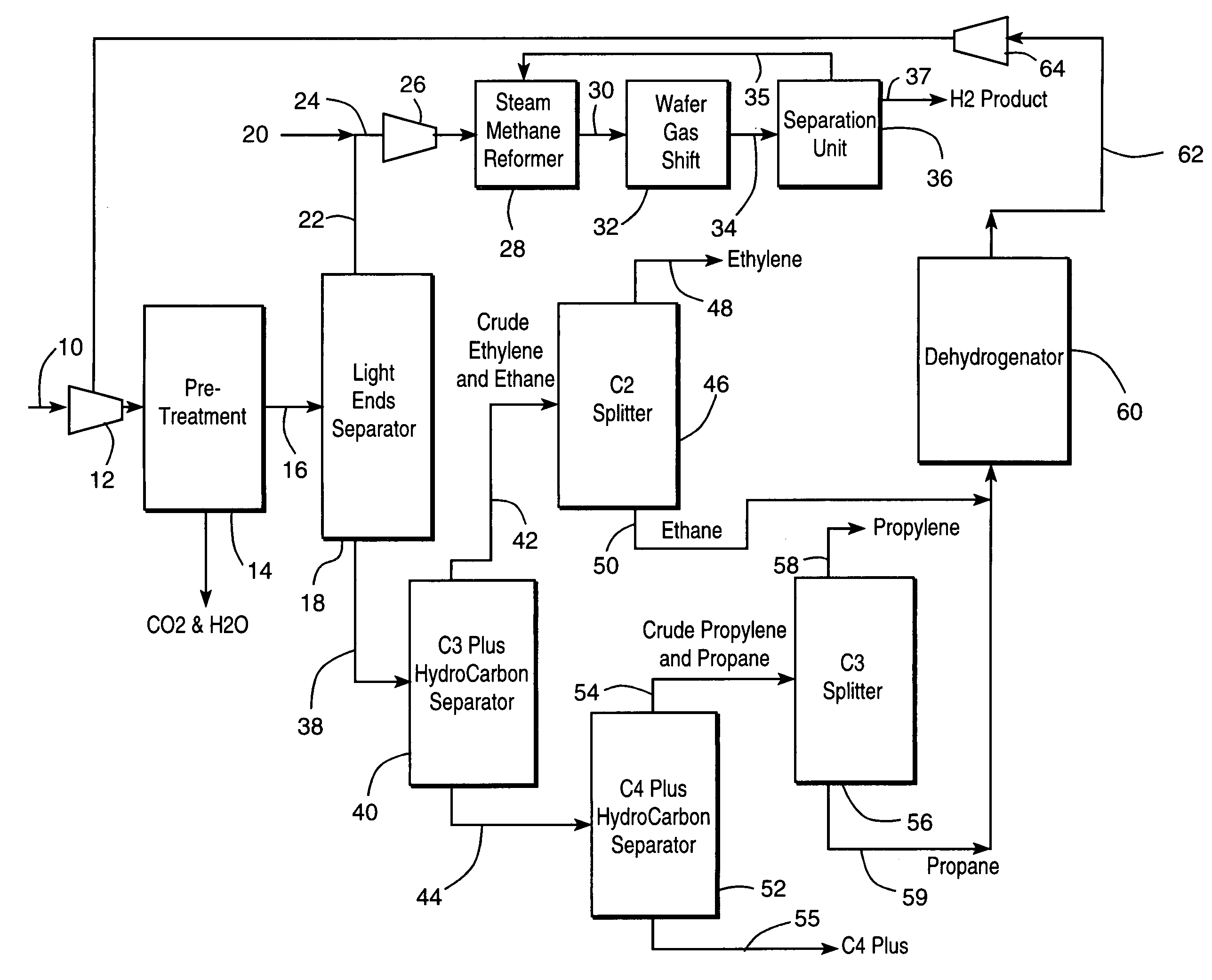
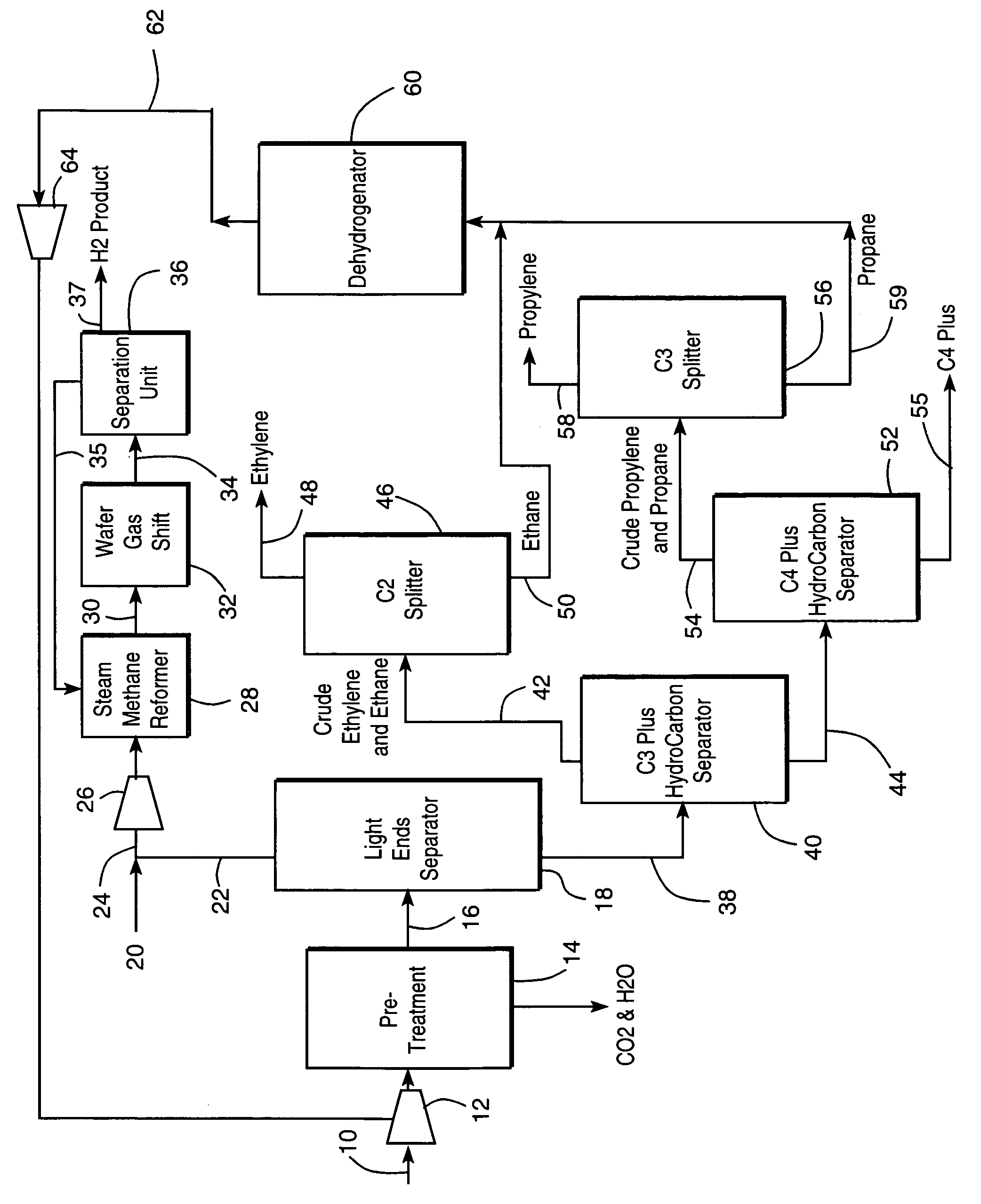
Integrated olefin recovery and hydrogen production from refinery off-gases
A method is disclosed a method for recovering olefins and for producing hydrogen from a refinery off-gas stream in which such stream is conventionally pretreated and separated to obtain a light ends stream that contains nitrogen, hydrogen and carbon monoxide and a heavy ends stream that contains the olefins. The light ends stream is subjected to reforming and a water gas shift reactions after addition of a natural gas stream. The addition of the natural gas increases the hydrogen recovery from the light ends and also stabilizes the hydrocarbon content in the stream to be subjected to the reforming and water gas shift reactions. The heavy ends can be further treated to recover olefins such as ethylene and propylene. The rate of natural gas addition is controlled so that the concentration of the nitrogen in a stream exiting the water gas shift reactor is less than about 5 percent by volume so that hydrogen separation from such stream becomes practical.
Owner:PRAXAIR TECH INC
Catalyst having metal in reduced quantity and reduced cluster size
ActiveUS20060128565A1Reduce the amount requiredRetain activityHydrogenCatalyst activation/preparationPlatinumWater-gas shift reaction
The invention contemplates a method of making a catalytic material, and uses of the material. The catalytic material is made by depositing catalytic metals, such as gold or platinum, on substrate materials, such as lanthanum-doped ceria or other oxides. The catalytic metal, which comprises both crystalline and non-crystalline structures, is treated, for example with aqueous basic NaCN solution, to leach away at least some of the crystalline metallic component. The remaining noncystalline metallic component associated with the substrate exhibits catalytic activity that is substantially similar to the catalyst as prepared. The use of the catalyst in an apparatus such as a reactor or analytic instrument is contemplated, as is the use of the catalyst in efficient, cost-effective reactions, such as removal of carbon monoxide from fuel gases, for example by performing the water gas shift reaction.
Owner:TUFTS UNIV
Integration of mixed catalysts to maximize syngas production
Embodiments include a method and apparatus for converting a hydrocarbon and oxygen feed stream to a product stream such as syngas, including multiple serially aligned reaction zones and multiple hydrocarbon feeds. The first reaction zone catalyzes the net partial oxidation of the feed hydrocarbon. The subsequent zones catalyze reactions such as the stream or dry reforming of hydrocarbons or the water gas shift reaction, depending on the stream composition in the vicinity of the zone, and the desired product stream composition.
Owner:CONOCOPHILLIPS CO
Production of dry alcohol
ActiveUS20070238906A1Improve energy efficiencyLiberating heatHydrogenOrganic compound preparationAlcoholWater-gas shift reaction
A process for producing dry alcohol includes at least one stage wherein a gaseous feedstock, which includes alcohol and water, is contacted with carbon monoxide in the presence of a water-gas shift catalyst, at a temperature sufficiently high so that carbon monoxide and water are consumed and carbon dioxide and hydrogen are produced, thereby removing a portion of the water. The process may include multiple stages; the dry alcohol produced contains 99.5 wt. % or greater of alcohol and 0.5 wt. % or less of water. A preferred alcohol is ethanol; dry ethanol can be combined with gasoline to afford a gasohol.The water-gas shift reaction is exothermic. The heat generated may be used to increase the energy efficiency of the process. In one embodiment, the heat is used to generate high-pressure steam. The high-pressure steam may be used, for example, to convert feedstock to gaseous feedstock or in the operation of a production plant.
Owner:RCM TECH USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com