Method for synthesizing ursodesoxycholic acid with chenodeoxycholic acid by photochemical method
A technique for chenodeoxycholic acid and ursodeoxycholic acid is applied in the field of preparation of ursodeoxycholic acid, can solve problems such as poor stereoselectivity, and achieve the effects of stable quality, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
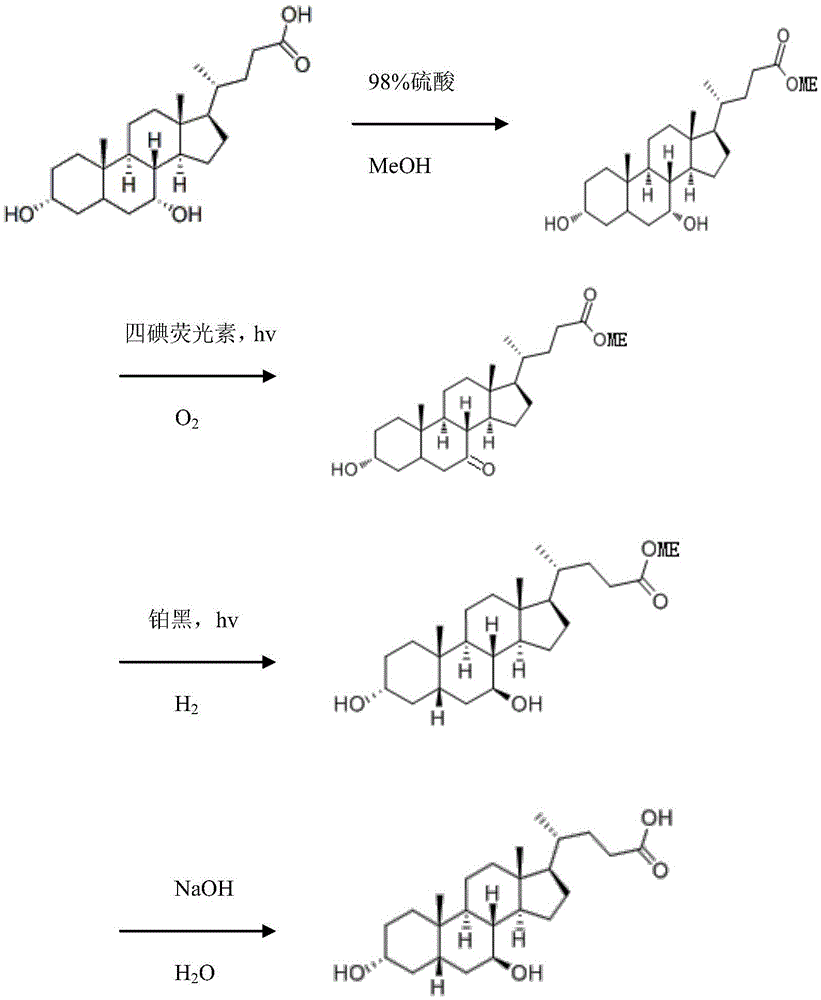
[0036] A kind of photochemical method uses chenodeoxycholic acid to synthesize the method for ursodeoxycholic acid, it comprises the following steps:
[0037] A, Preparation of methyl chenodeoxycholate
[0038] Take 39.2g of chenodeoxycholic acid (99.4% content) and dissolve it in 200mL of methanol, stir at room temperature until it dissolves completely, then slowly add 5mL of 98% concentrated sulfuric acid, stir at room temperature for 4 hours, and confirm the reaction is complete by spotting samples by thin-layer chromatography After adding 7.92g of sodium hydroxide, stirring and neutralizing sulfuric acid, when the pH was 6 to 8, the neutralization product sodium sulfate was removed by filtration; the filtrate was concentrated to obtain 41g of methyl chenodeoxycholic acid, which was determined by high performance liquid chromatography. Oxycholic acid methyl ester content is 97.3%;
[0039] B, Preparation of 3α-hydroxy-7-keto-5β-cholanoic acid methyl ester by photochemical ...
Embodiment 2
[0045] A kind of photochemical method uses chenodeoxycholic acid to synthesize the method for ursodeoxycholic acid, it comprises the following steps:
[0046] A, Preparation of methyl chenodeoxycholate
[0047]Take 39.2 g of chenodeoxycholic acid (99%) and dissolve it in 200 mL of methanol, stir at room temperature until it is completely dissolved, then slowly add 5 mL of 98% concentrated sulfuric acid, stir at room temperature for 4 hours, and confirm that the reaction is complete by applying thin-layer chromatography. Add sodium hydroxide, stir to neutralize sulfuric acid, and when the pH is 6 to 8, filter and remove the neutralized product sodium sulfate; Concentrate the filtrate to obtain 41.2g of chenodeoxycholic acid methyl ester, and determine chenodeoxychole Acid methyl ester content is 97%;
[0048] B, Preparation of 3α-hydroxy-7-keto-5β-cholanoic acid methyl ester by photochemical oxidation
[0049] Dissolve 40.6g of methyl chenodeoxycholic acid in 200mL of n-butan...
Embodiment 3
[0054] A kind of photochemical method uses chenodeoxycholic acid to synthesize the method for ursodeoxycholic acid, it comprises the following steps:
[0055] A, Preparation of methyl chenodeoxycholate
[0056] Take 39.2 g of chenodeoxycholic acid (99.2%) and dissolve it in 200 mL of methanol, stir at room temperature until it dissolves completely, then slowly add 5 mL of 98% concentrated sulfuric acid, stir at room temperature for 4 hours, and confirm that the reaction is complete by applying thin-layer chromatography Add sodium hydroxide, stir to neutralize sulfuric acid, and when the pH is 6 to 8, remove the neutralized product sodium sulfate by filtration; Concentrate the filtrate to obtain 41.3g of chenodeoxycholic acid methyl ester, and determine chenodeoxychole Acid methyl ester content is 97.1%;
[0057] B, Preparation of 3α-hydroxy-7-keto-5β-cholanoic acid methyl ester by photochemical oxidation
[0058] Dissolve 40.6g of methyl chenodeoxycholic acid in 200mL of n-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com