Method for synthesizing UDCA (ursodesoxycholic acid) by catalyzing CA (cholic acid) by chemical cells
A cell and chemical technology, applied in the field of chemical cell catalysis of CA to synthesize UDCA, can solve the problems of expensive coenzyme, incomplete reaction, strict storage conditions, etc., and achieve simplified catalytic steps and catalytic conditions, simple reaction steps and conditions, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
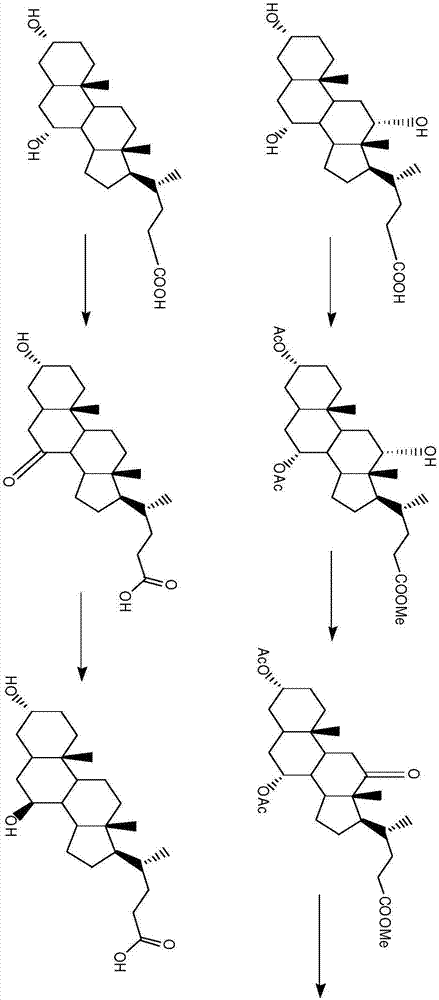
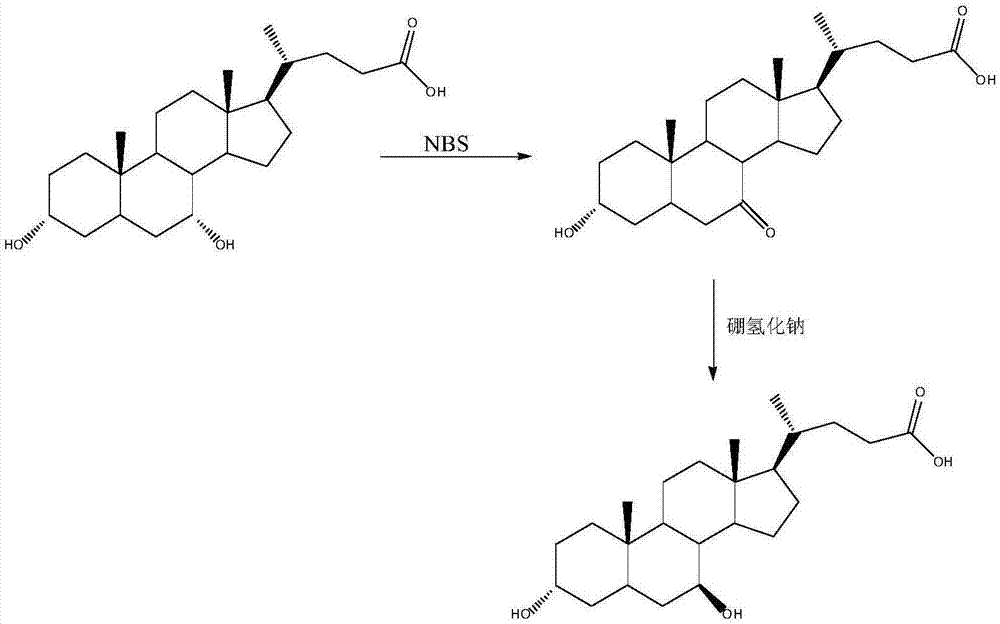
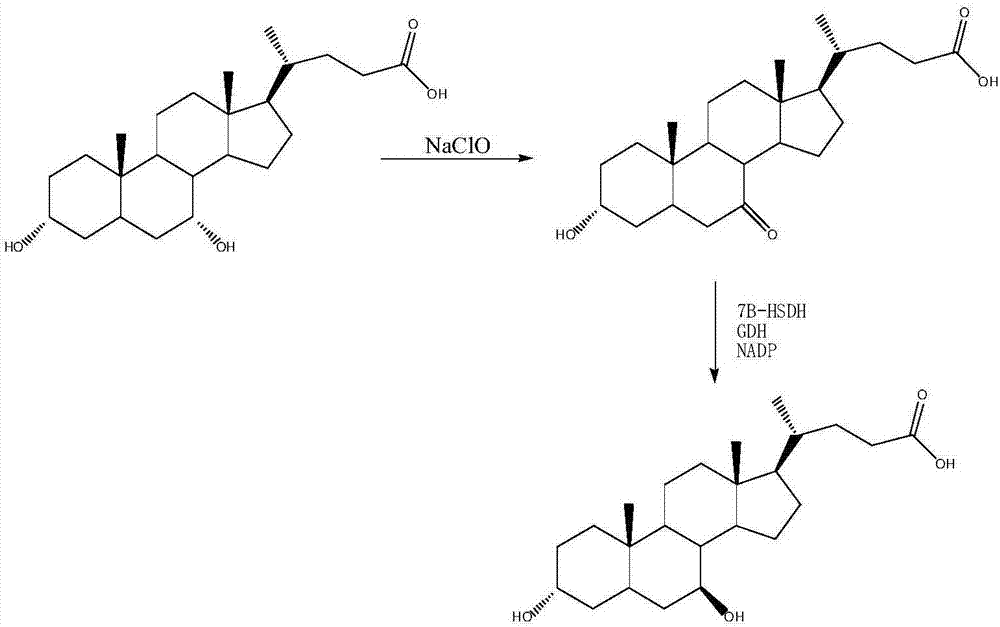
Image
Examples
Embodiment 1-4
[0029] Example 1-4 is the construction of 3α-HSDH-7β-HSHD-GDH recombinant plasmid transformed into BL21 (DE3)
Embodiment 1
[0031] 3α-HSDH from Clostridium perfingens, 7β-HSDH from Bacteroides fragilis and GDH from Agrobacterium Tumefaciens were co-constructed. The gene was constructed on the plastid vector in a tandem manner, and then transformed into E. coli cells to obtain the recombinant strain SDM109.
Embodiment 2
[0033] 3α-HSDH from Eubacterium lentum, 7β-HSDH from Collinsella aerofaciens and GDH from Bacillus Anthracis were co-constructed. These three genes were chromosomally Integration, and then transformed into E. coli cells to obtain recombinant strain SDM109.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com