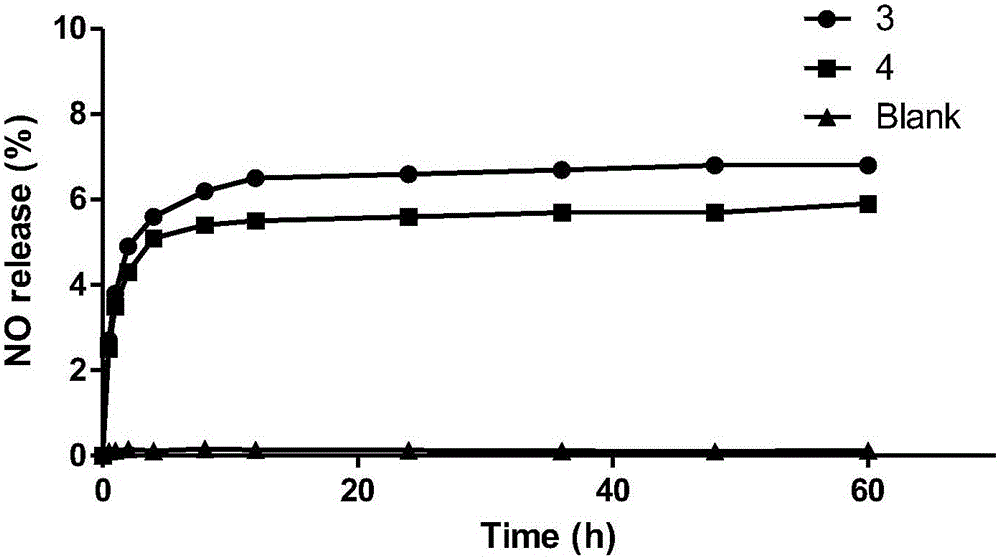
Platinum (II) coordination complex and preparing method and application thereof
A technology of complexes and drugs, which is applied in platinum-group organic compounds, platinum-based organic compounds, pharmaceutical formulations, etc., can solve problems such as cross-resistance, toxic and side effects, and achieve mild release activity and good in vitro inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
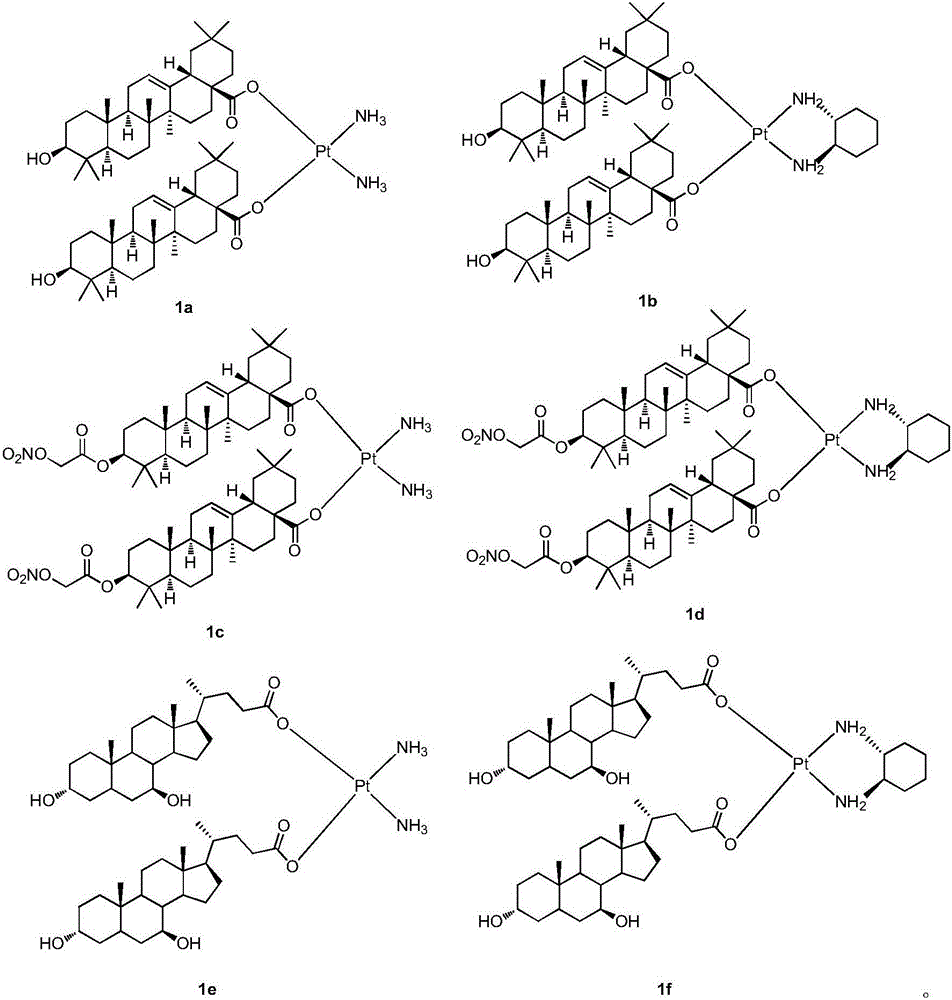
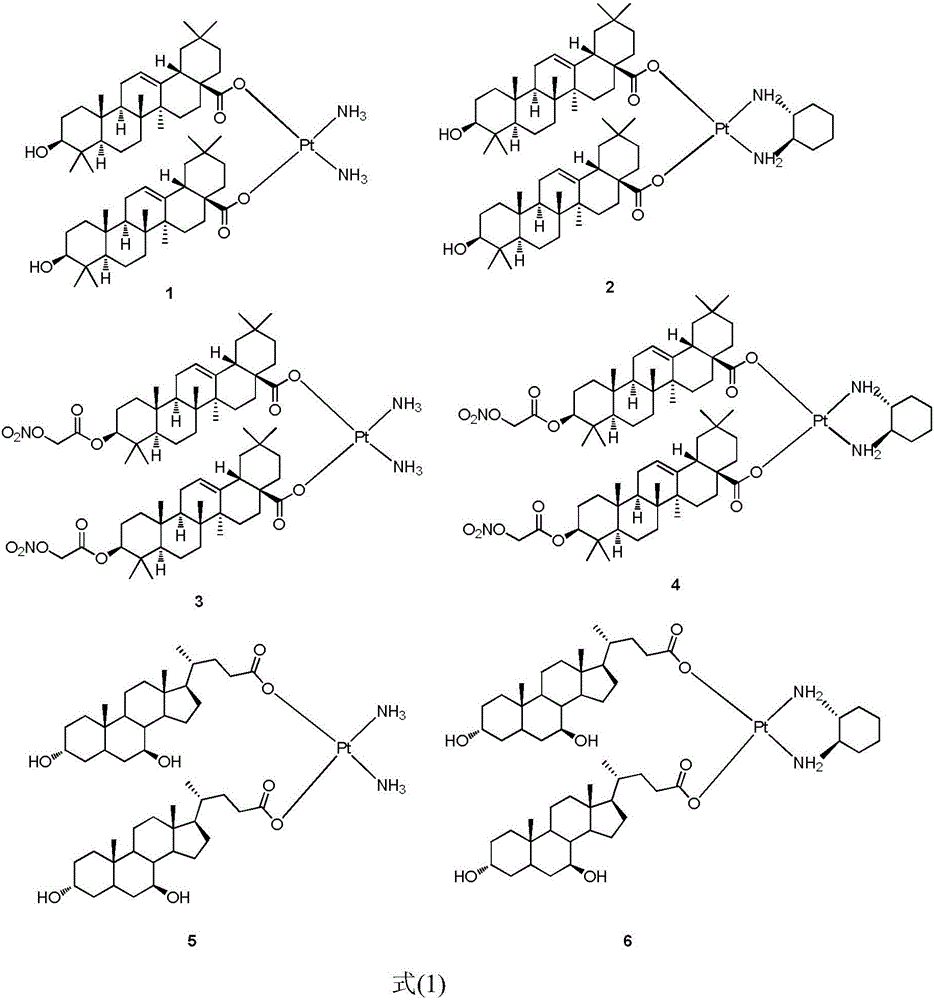
[0015] The preparation method of novel platinum (II) complex of the present invention is shown in reaction formula (I):
[0016]
[0017] Potassium tetrachloroplatinate coordinates with ammonia or (R,R)-cyclohexanediamine in the presence of potassium iodide to obtain intermediate 2 or 2', and then removes iodide ion by silver nitrate under light-shielding conditions, and then reacts with The complex represented by the formula (1) is obtained by reacting the sodium salt of ursodeoxycholic acid or oleanolic acid or an oleanolic acid derivative.
[0018] The pharmaceutical use of the novel platinum (II) complexes of the present invention is that the novel platinum (II) complexes are used for the treatment of malignant tumors, and they can treat human liver cancer cells HepG2, human gastric cancer cells SGC7901, human lung cancer cells A549 and human Colon cancer cell HCT-116 showed good inhibitory activity in vitro.
Embodiment 1
[0019] Embodiment 1. Preparation of oleanolic acid diammine platinum (compound 1)
[0020]
[0021] Diiododiammine platinum (0.5g, 1mmol) and silver nitrate (0.3g, 2mmol) were dissolved in 100ml of water, stirred and reacted at 40°C for 24h in the dark, and the clear solution was filtered. Then add the prepared tetrahydrofuran / water mixed solution of sodium oleanolic acid (1.0g, 2mmol) into the above reaction solution, stir and react in the dark at 40°C for 24h, filter, concentrate the filtrate to about 10ml, cool, and have a white The solid was precipitated, filtered, and recrystallized from tetrahydrofuran to obtain a white product with a yield of 40%. 1 HNMR (DMSO-d 6 ,500MHz):δ5.30-5.28(m,2H,2C H =C),4.20(br,6H,2N H 3 ),3.24-3.18(m,2H,2C 3 - H ),2.90-2.86(m,2H,2C 18 - H ),1.14(s,6H,2C H 3 ),0.99(s,6H,2C H 3 ),0.93(s,6H,2C H 3 ),0.91(s,6H,2C H 3 ),0.90(s,6H,2C H 3 ),0.78(s,6H,2C H 3 ),0.72(s,6H,2C H 3 )cm -1 ;ESI-MS:1140.5[M+H] + .
Embodiment 2
[0022] Embodiment 2. Preparation of oleanolic acid cyclohexanediamine platinum (compound 2)
[0023]
[0024] Prepared by reacting diiodocyclohexanediamine platinum with oleanolic acid sodium salt in the presence of silver nitrate, the method is the same as that of Example 1, and the yield is 29%. 1 HNMR (DMSO-d 6 ,500MHz):δ5.96(br,2H,N H 2 ),5.30-5.28(m,2H,2C H =C),5.25(br,2H,N H 2 ),3.23-3.19(m,2H,2C 3 - H ),2.91-2.86(m,2H,2C 18 - H ),1.14(s,6H,2C H 3 ),0.99(s,6H,2C H 3 ),0.93(s,6H,2C H 3 ),0.91(s,6H,2C H 3 ),0.90(s,6H,2C H 3 ),0.78(s,6H,2C H 3 ),0.72(s,6H,2C H 3 )cm -1 ;ESI-MS: 1250.7[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com