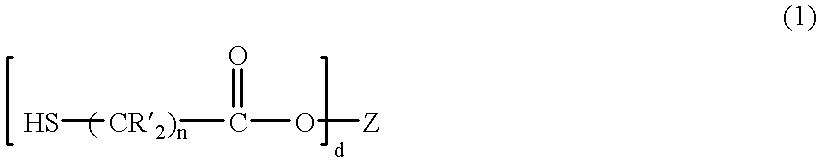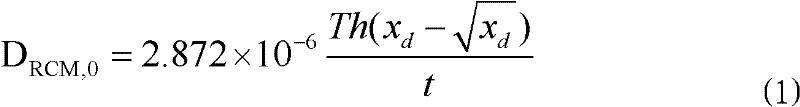Patents
Literature
424 results about "Iodide ion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An iodide ion is the ion I −. Compounds with iodine in formal oxidation state −1 are called iodides . This page is for the iodide ion and its salts, not organoiodine compounds .
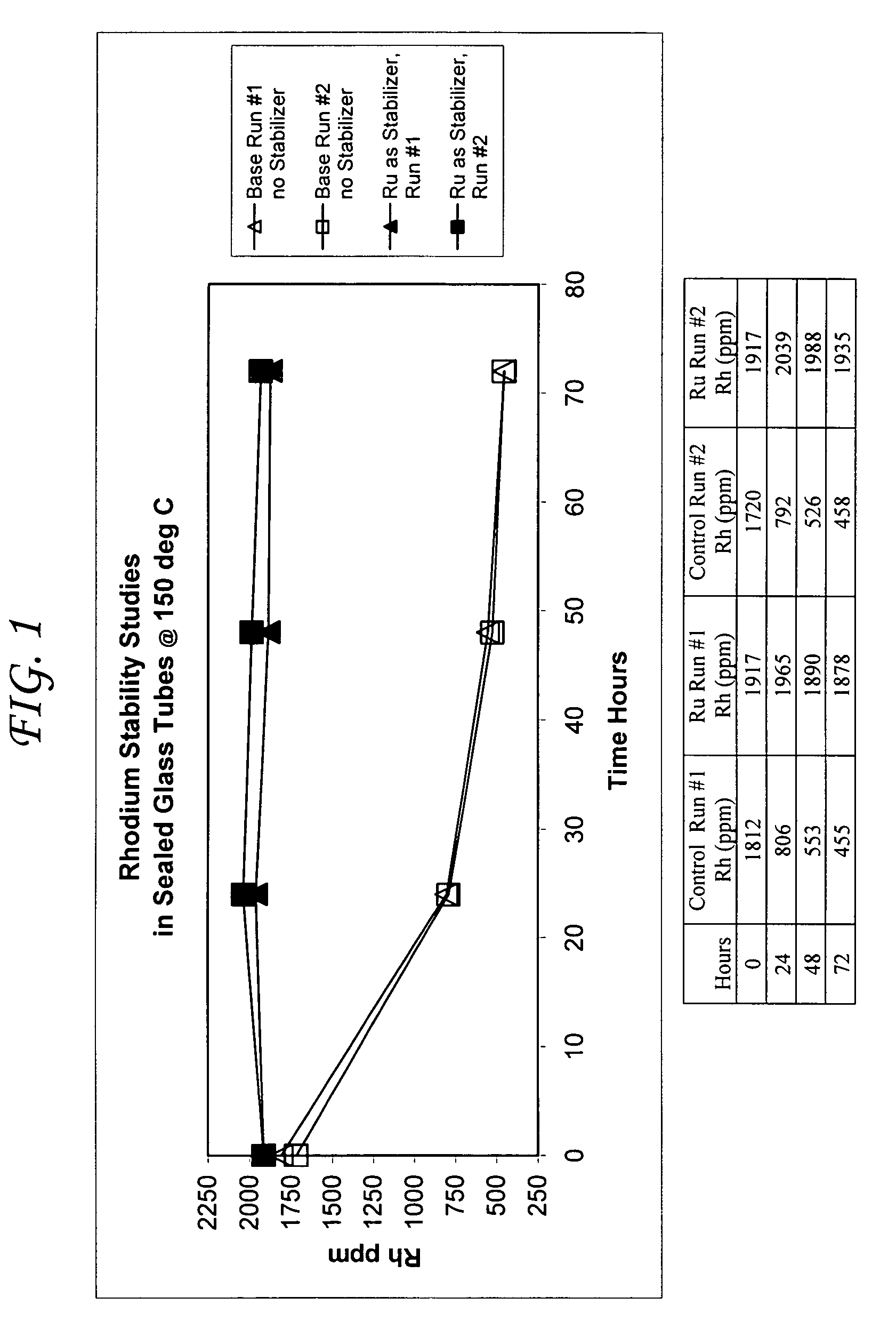
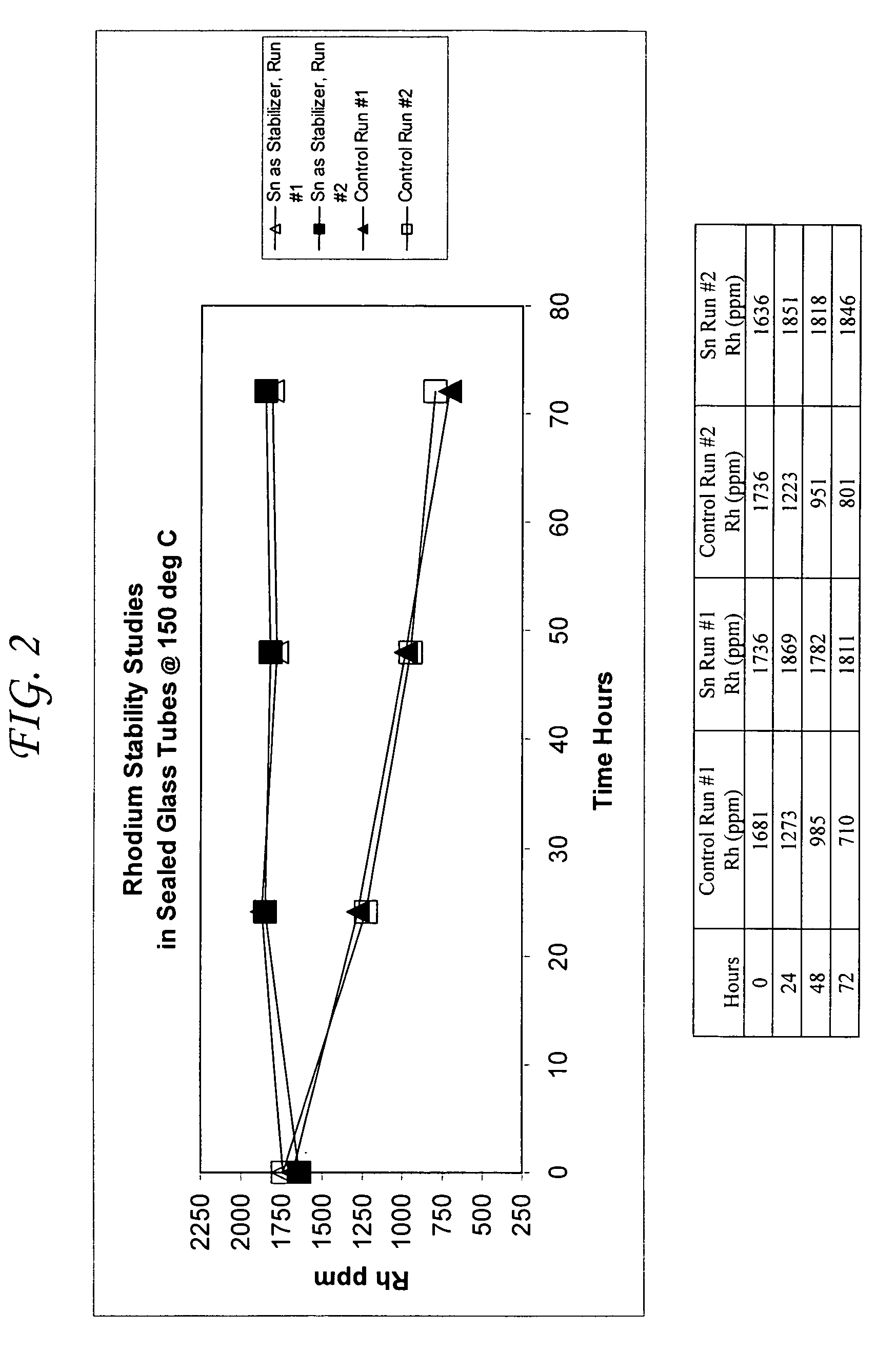
Addition of iridium to the rhodium/inorganic iodide catalyst system
InactiveUS6211405B1High rateReduce productionPhysical/chemical process catalystsOrganic compound preparationPtru catalystCarboxylic acid
The present invention provides a process for the carbonylation of an alcohol, ether or ester to products comprising a carboxylic acid, the anhydride thereof or coproduction of the carboxylic acid and anhydride. More particularly, the present invention provides a process for the carbonylation of methanol to produce acetic acid by reacting methanol with carbon monoxide in a liquid reaction medium containing a catalyst comprising rhodium, iridium, iodide ion, and said reaction medium further comprising water, acetic acid, methyl iodide, and methyl acetate and subsequently recovering acetic acid from the resulting reaction product.
Owner:CELANESE INT CORP
Magnesium ion-containing non-aqueous electrolyte and a production process thereof, as well as electrochemical device
ActiveUS20090068568A1Effective resourcesEfficient use ofCell electrodesOrganic electrolyte cellsAluminum IonPhosphate ion
A magnesium ion containing non-aqueous electrolyte in which magnesium ions and aluminum ions are dissolved in an organic etheric solvent, and which is formed by: adding metal magnesium, a halogenated hydrocarbon RX, an aluminum halide AlY3, and a quaternary ammonium salt R1R2R3R4N+Z− to an organic etheric solvent; and applying a heating treatment while stirring them (in the general formula RX representing the halogenated hydrocarbon, R is an alkyl group or an aryl group, X is chlorine, bromine, or iodine, in the general formula AlY3 representing the aluminum halide, Y is chlorine, bromine, or iodine, in the general formula R1R2R3R4N+Z− representing the quaternary ammonium salt, R1, R2, R3, and R4 represent each an alkyl group or an aryl group, and Z− represents chloride ion, bromide ion, iodide ion, acetate ion, perchlorate ion, tetrafluoro borate ion, hexafluoro phosphate ion, hexafluoro arsenate ion, perfluoroalkyl sulfonate ion, or perfluoroalkyl sulfonylimide ion.
Owner:MURATA MFG CO LTD
Skin dressings
InactiveUS20050181026A1Helpful background level of excess moistureEfficient workBiocideCarbohydrate active ingredientsMedicineDermal dressing
Owner:INSENSE LIMITED
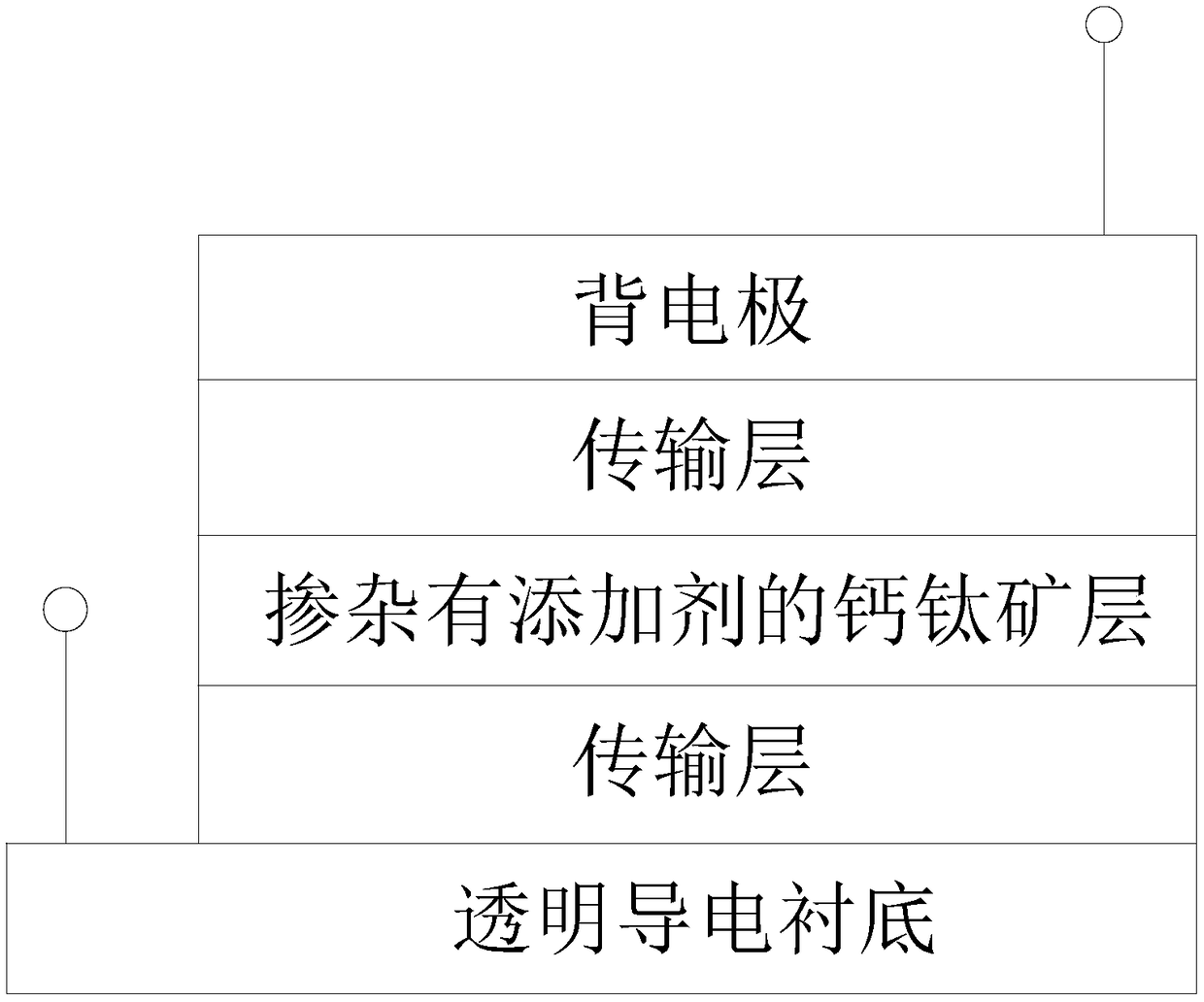
Additive-doped perovskite film and preparation method and application thereof
InactiveCN108321300AImprove long-term stabilityExtended service lifeSolid-state devicesSemiconductor/solid-state device manufacturingHalogenIodine
The invention relates to an additive-doped perovskite film. The perovskite film is doped with an additive which is a stabilizer formed by metal ions M and halogen ions G. The invention also disclosesa preparation method and application of the additive-doped perovskite film. By incorporating an appropriate amount of additive during the preparation process of the perovskite film, the additive suppresses the movement of iodide ions in the perovskite film material, thereby stabilizing the material itself. Thus, a prepared perovskite battery is improved in long-term stability, performance, and service life, and industrial production is also promoted.
Owner:HANGZHOU MICROQUANTA SEMICON CO LTD
Skin dressings
InactiveUS20070148117A1Improve skinGood effectCosmetic preparationsToilet preparationsMedicineDermal dressing
Owner:INSENSE LIMITED
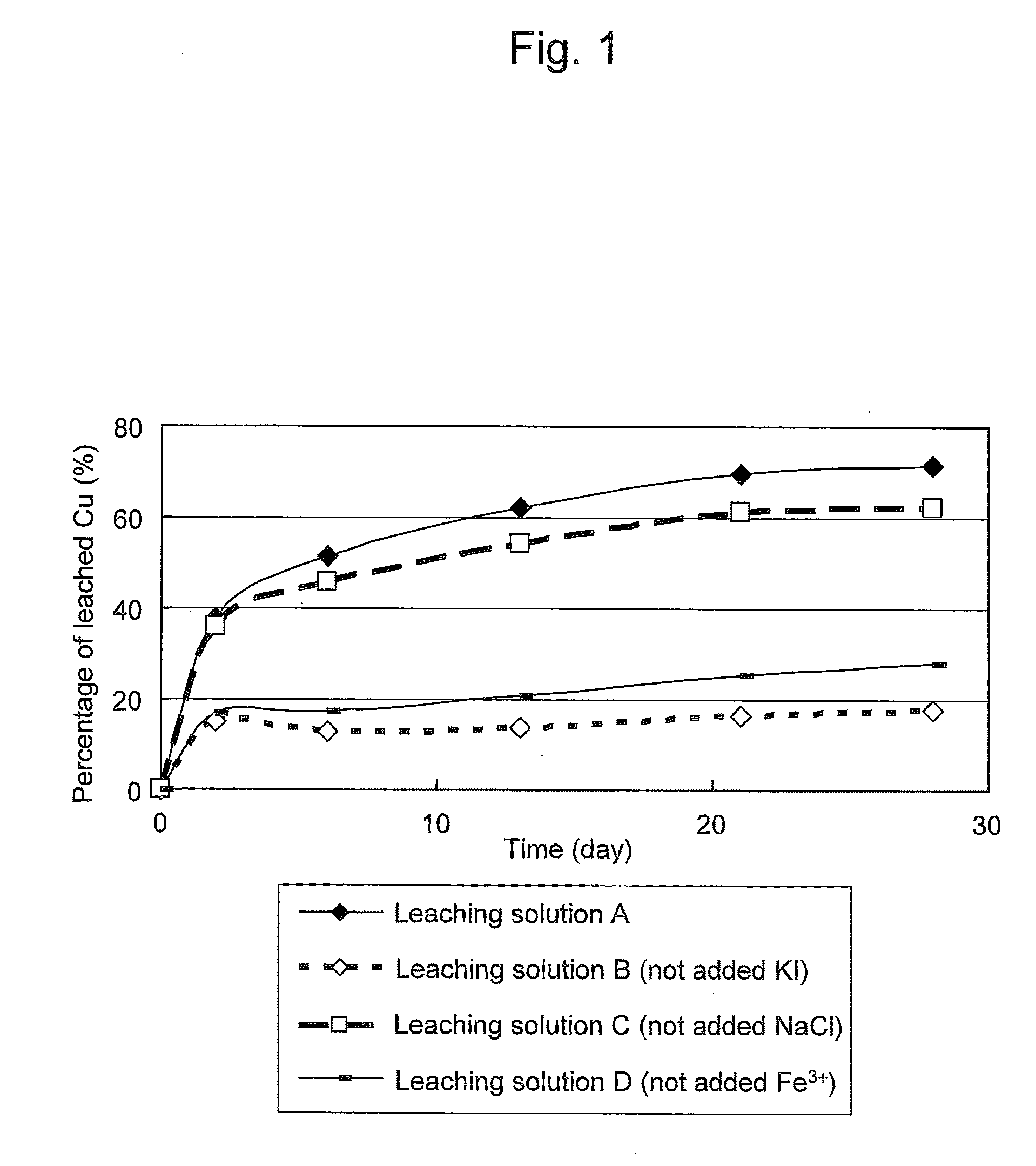
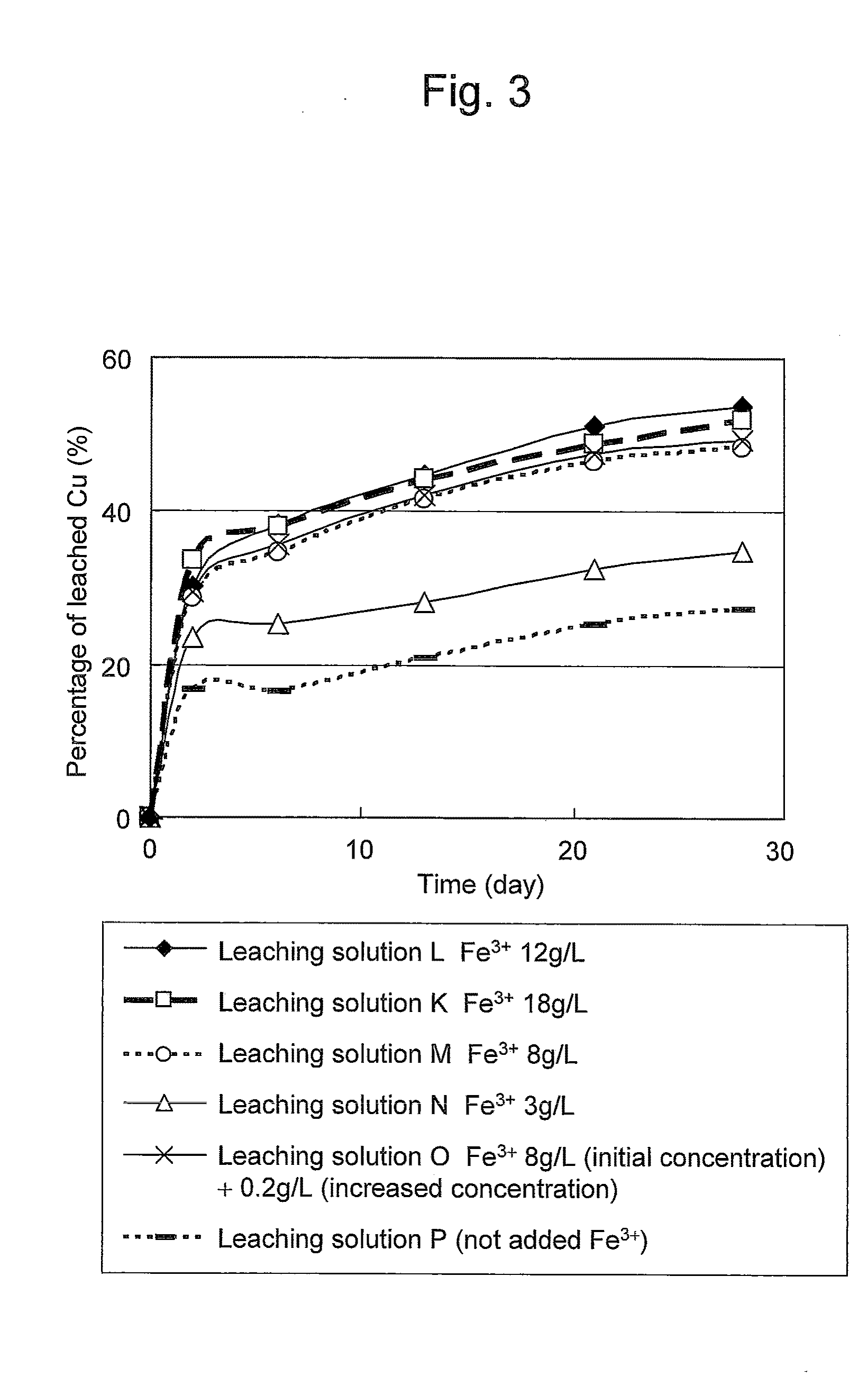
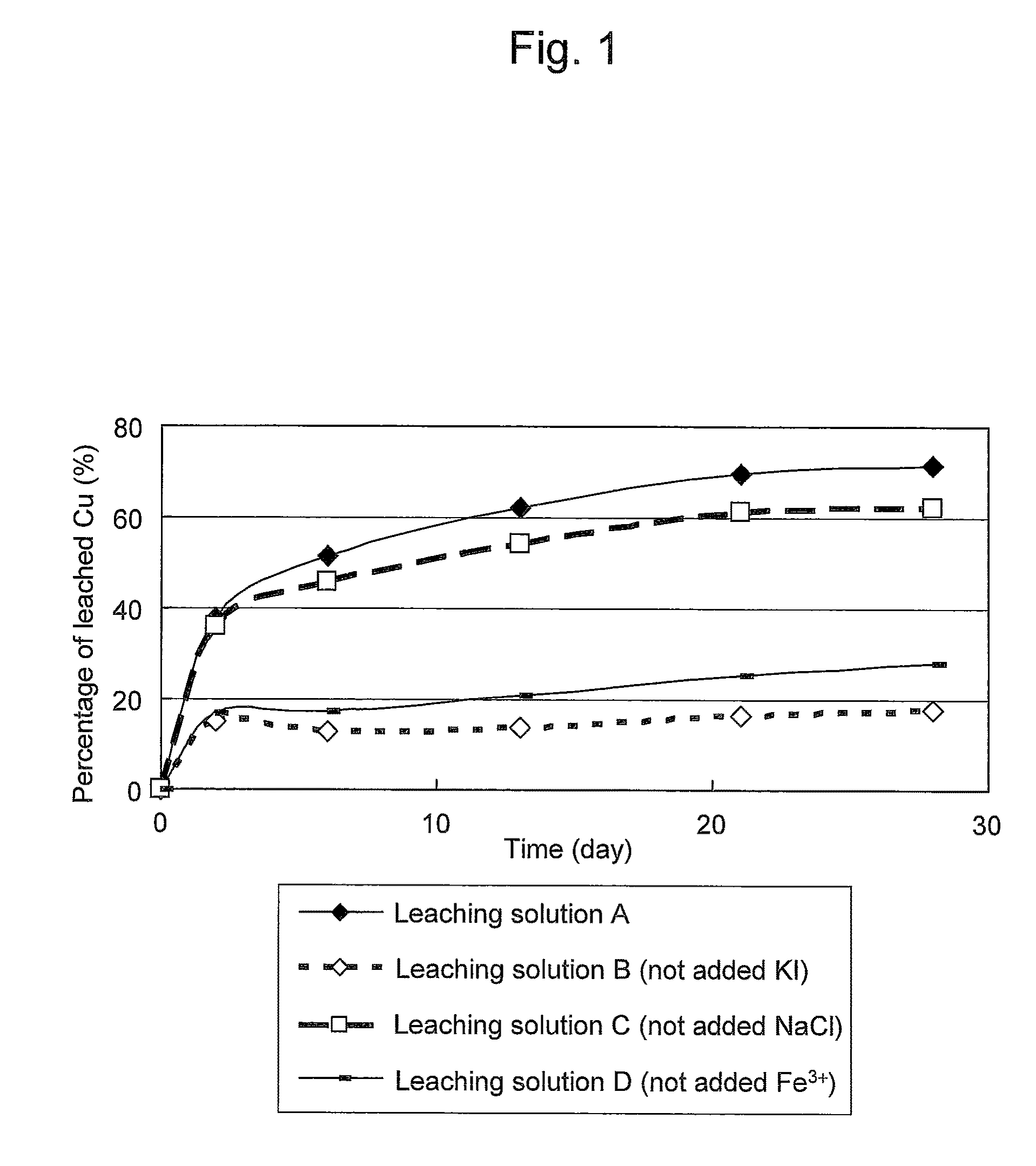
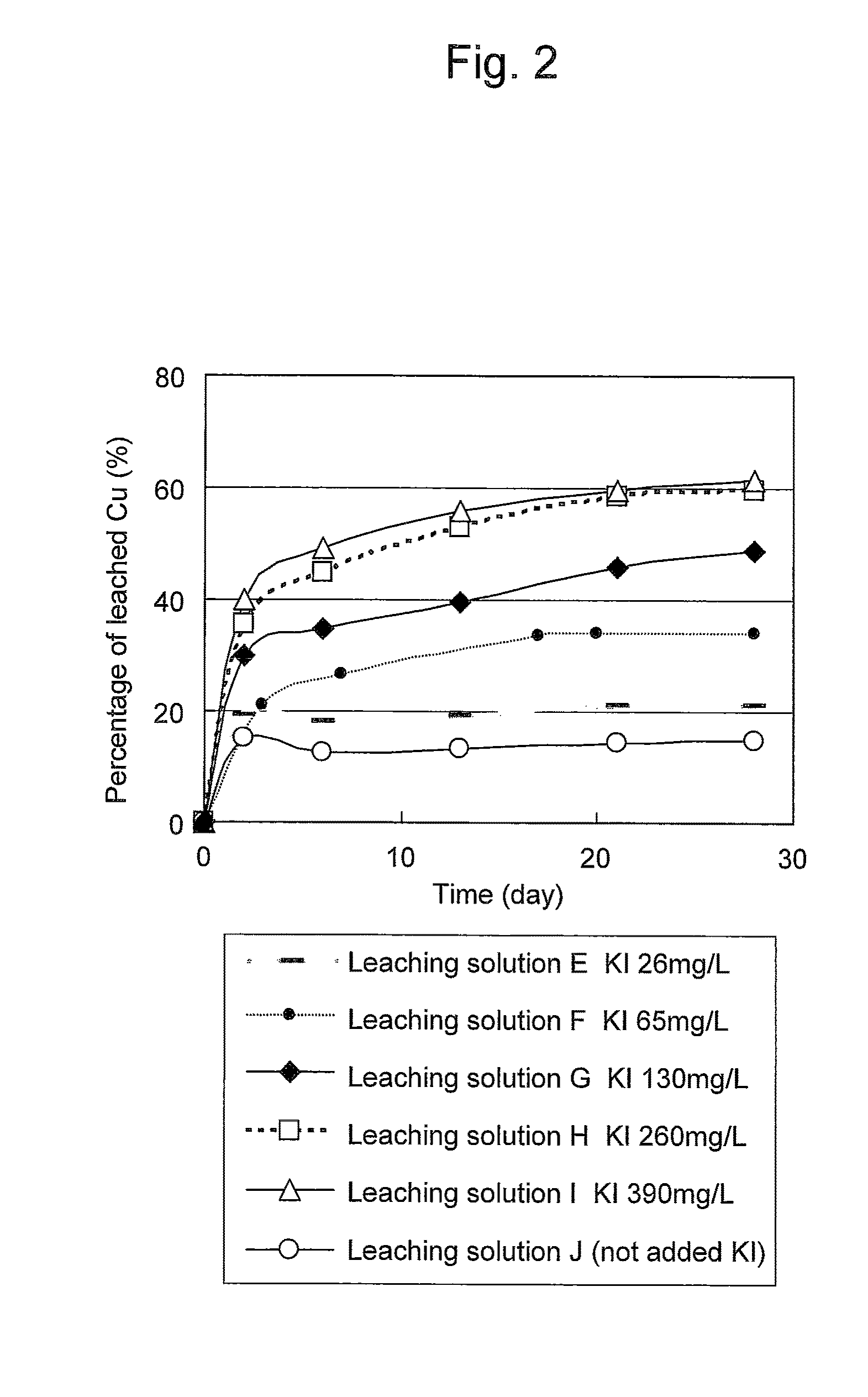
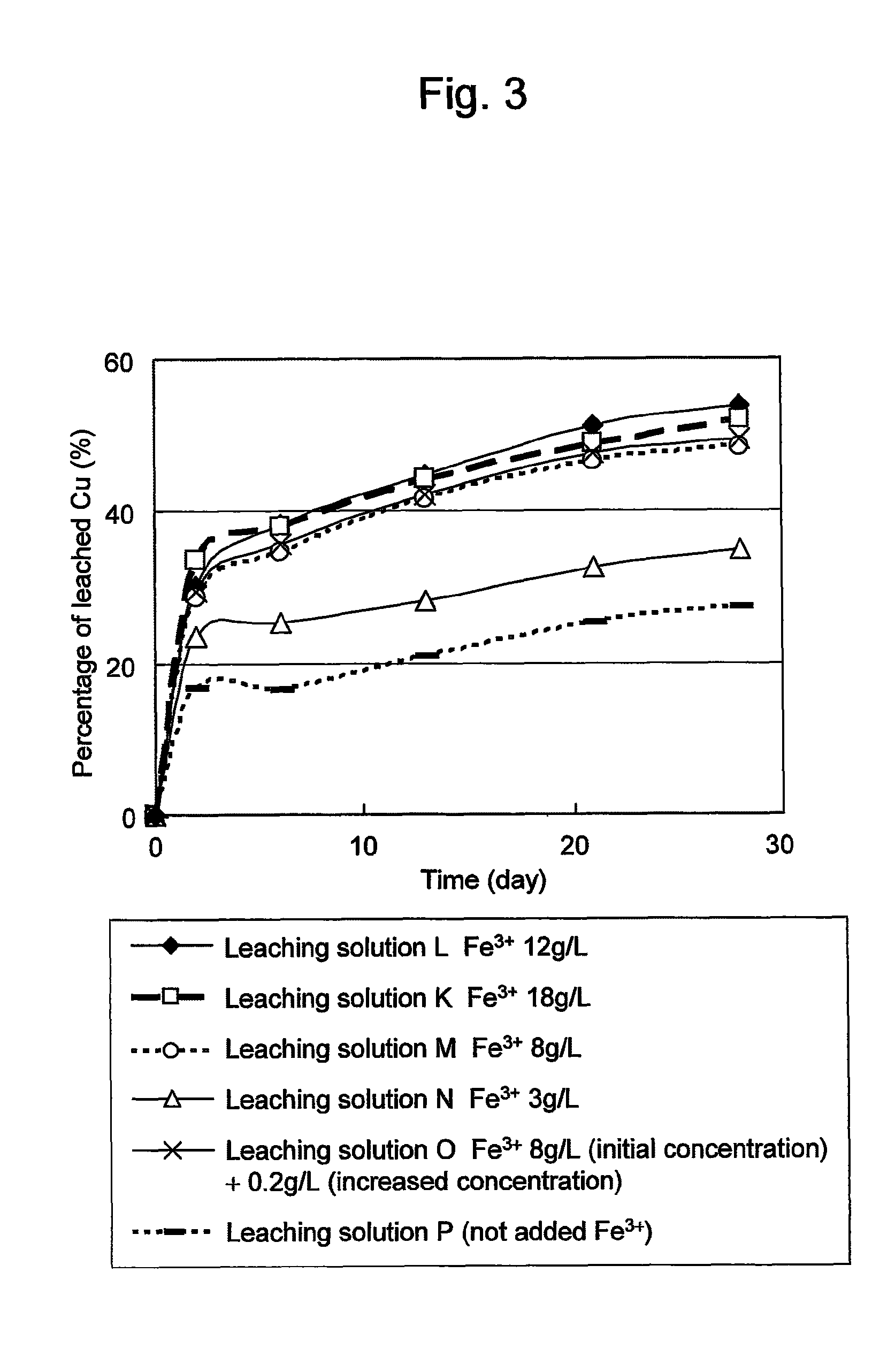
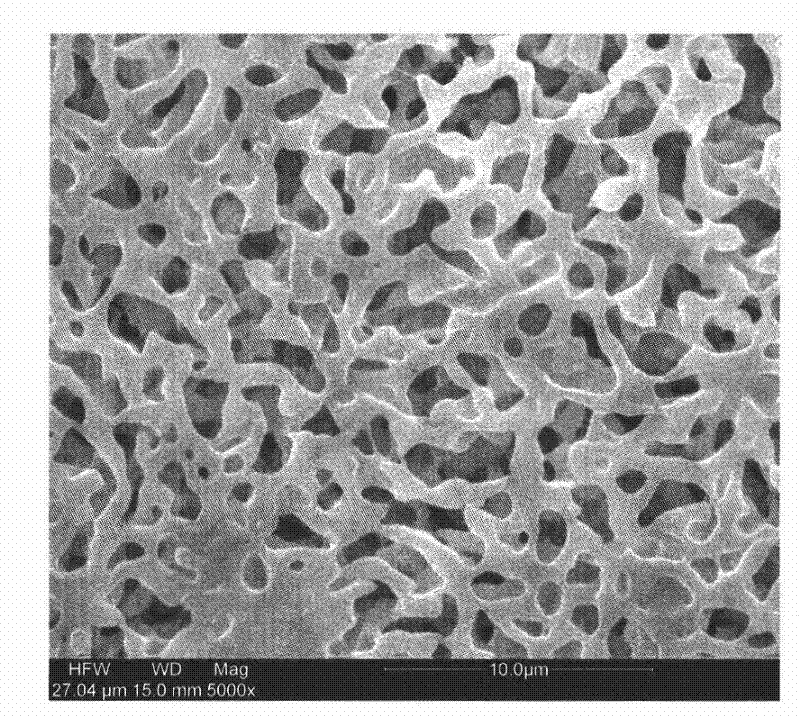
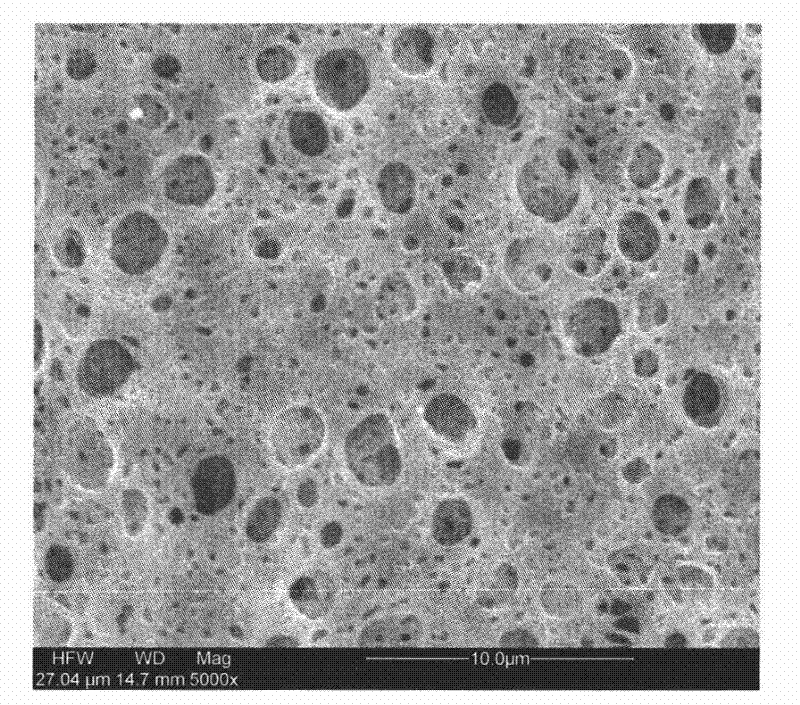
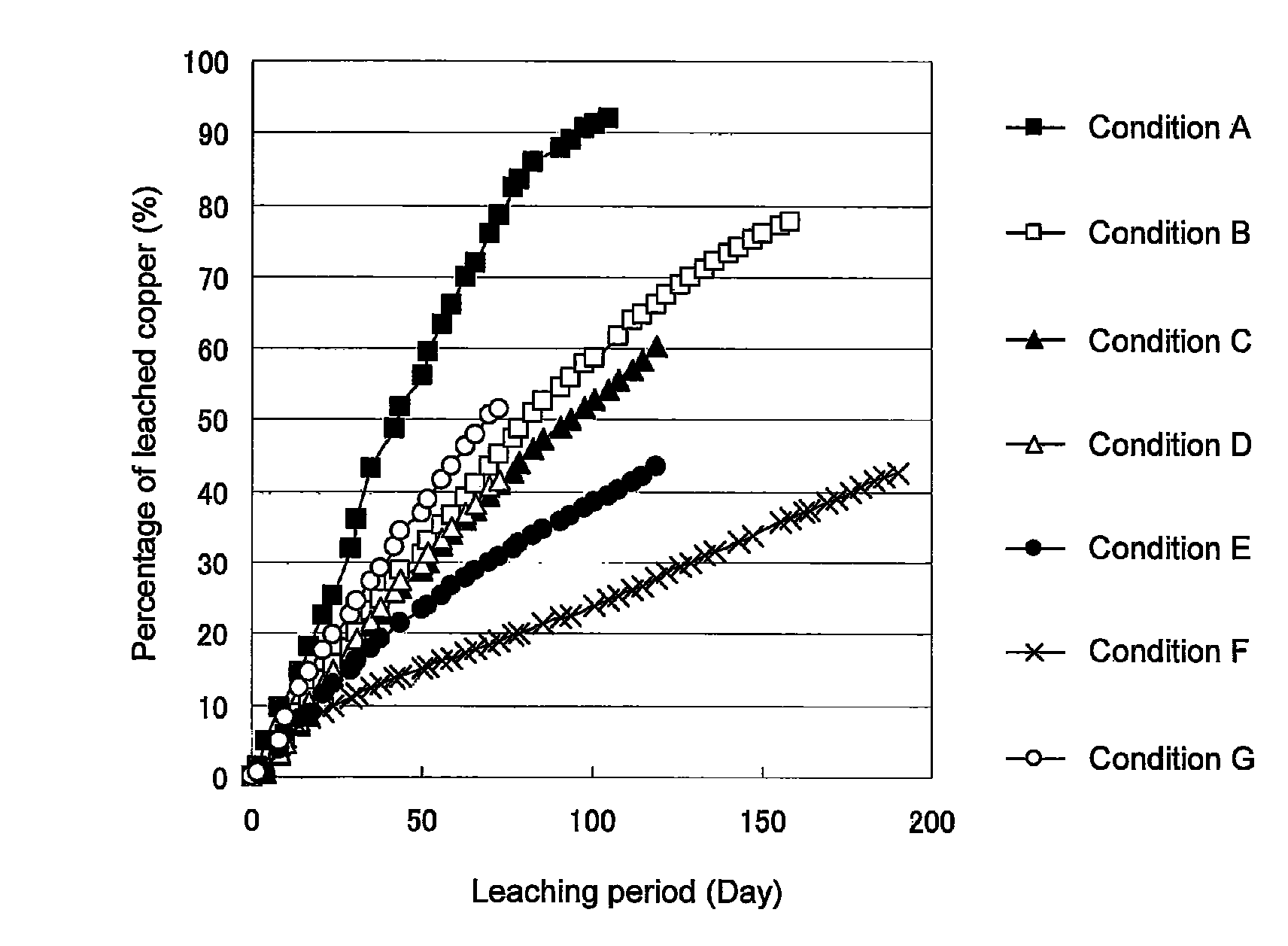
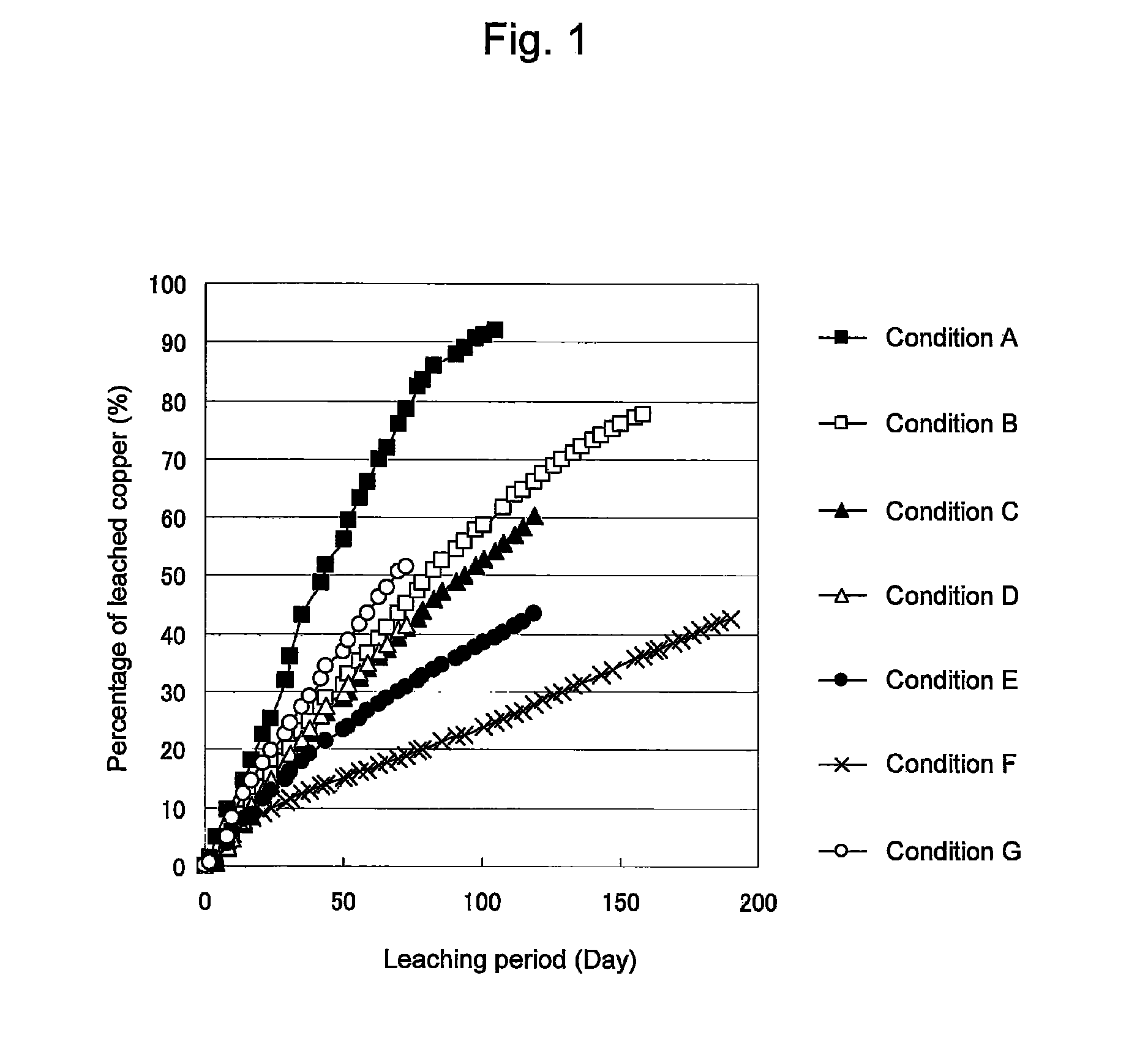
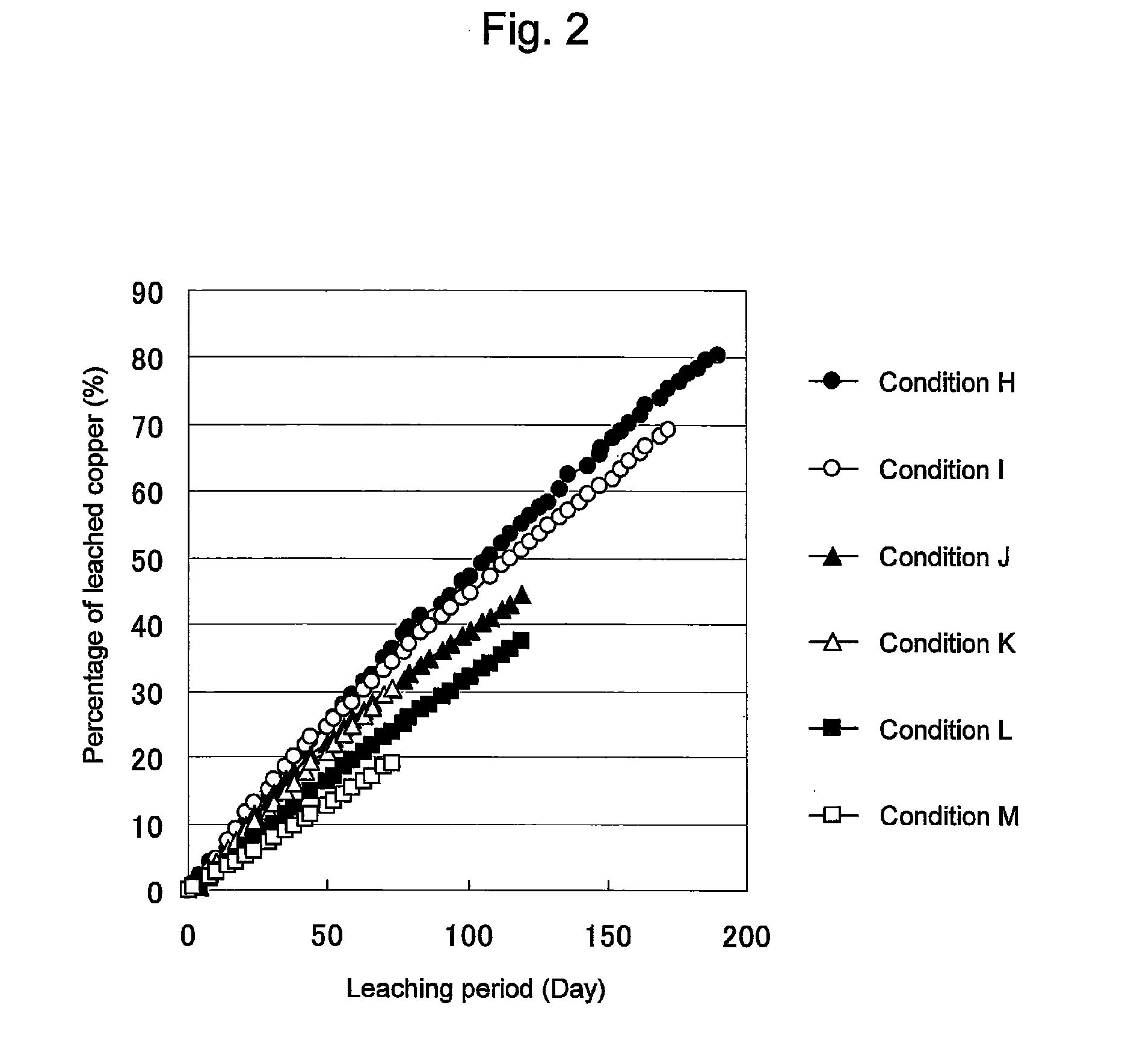
Method of leaching copper sulfide ore with the use of iodine
ActiveUS20100018349A1Efficient leachingEfficient executionSolvent extractionGold compoundsPregnant leach solutionChalcopyrite
An object of the present invention is to provide a method of efficiently leaching copper from a copper sulfide ore containing chalcopyrite or enargite as a main constituent under versatile conditions for actual operation.A method of leaching copper from a copper sulfide ore, characterized by comprising using, as a leaching solution, a sulfuric acid solution containing iodide ions and ferric (III) ions in an excessive amount relative to the iodide ions and leaching copper from a copper sulfide ore; or a method of leaching copper from a copper sulfide ore, characterized by comprising leaching copper from a copper sulfide ore with the use of a leaching solution further containing water-soluble ligands such as chloride ions that can stabilize ferric (III) ions in addition to the above components, is provided.
Owner:JX NIPPON MINING& METALS CORP
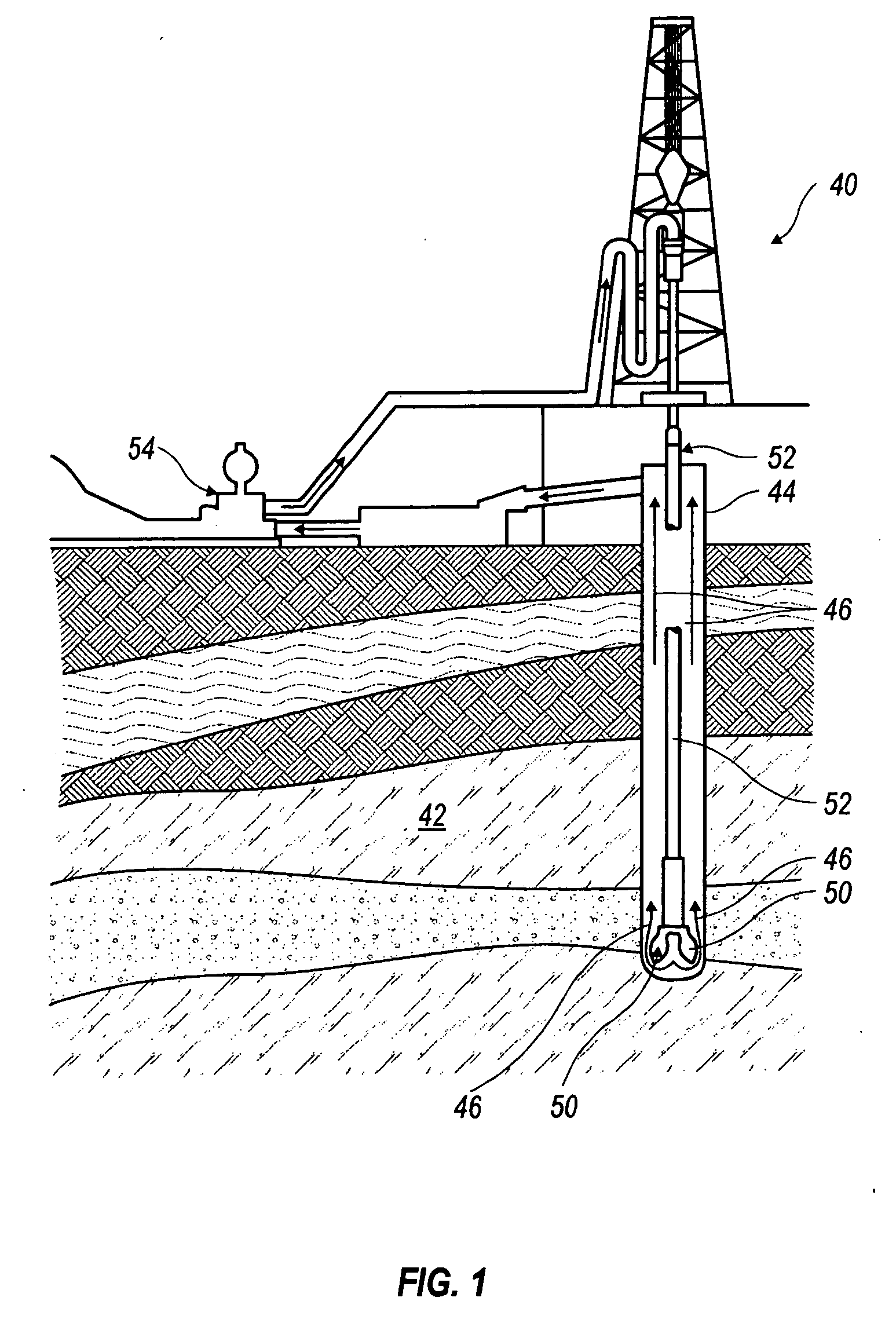
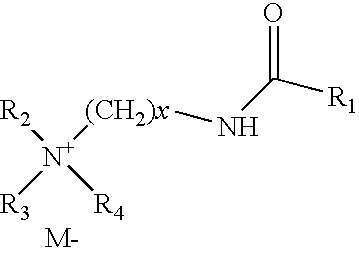
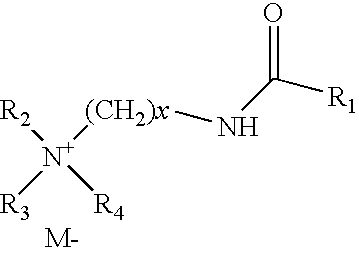
Drilling fluids containing biodegradable organophilic clay
Drilling fluids are provided that comprise an organophilic clay treated with a quaternary ammonium surfactant having an amide linkage. The quaternary ammonium surfactant may comprise a compound generally represented by the following formula: where M− is an anion such as a chloride, methyl sulfate, bromide, acetate, or iodide ion; R1 is an alkyl group such as a saturated hydrocarbon with 10 or more carbons; R2, R3, and R4 are the same or different alkyl groups such as a methyl, ethyl, or benzyl group, and x is greater than or equal to 1. The organophilic clay treated in this manner is substantially biodegradable. In embodiments, the drilling fluids comprise the foregoing organophilic clay, an oil-based fluid and a weighting agent. In still embodiments, the drilling fluids comprise the foregoing organophilic clay, an invert emulsion, an emulsifier, and a weighting agent.
Owner:HALLIBURTON ENERGY SERVICES INC
Ion liquid electrolyte containing bi-(fluorosulfonic acid) imines ion and iodine ion, and application thereof
ActiveCN101436467ALight-sensitive devicesPhotovoltaic energy generationPhysical chemistryFluorosulfonic acid
The invention relates to an ion liquid electrolyte containing bi-(fluorosulfonic acid)imine ion and iodine ion and application thereof to a dye sensitized solar battery. The electrolyte is a composition prepared from the bi-(fluorosulfonic acid)imine ion([N(SO2F)2]<->), iodine ion liquid and simple substance iodine which are evenly mixed according to the blending ratio, or the bi-(fluorosulfonic acid)imine ion([N(SO2F)2]<->), the iodine ion liquid, the simple substance iodine and one or more than one of other ion liquid, additive, curing agent and solvent which are evenly mixed according to the blending ratio. When the ion liquid electrolyte is applied to the dye sensitized solar battery, a high-efficiency thermal stability device with photoelectric power conversion efficiency of 7.5 percent is obtained, thereby the ion liquid electrolyte containing TCB negative ion is not used.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Acetic acid production methods incorporating at least one metal salt as a catalyst stabilizer
ActiveUS7053241B1Organic compound preparationCarboxylic preparation from carbon monoxide reactionAccelerantProtein carbonyl
Processes for the production of acetic acid by carbonylation of methanol, and reactive derivatives thereof, in a reaction mixture using a rhodium-based catalyst system with at least one metal salt catalyst stabilizer selected from the group of ruthenium salts, tin salts, and mixtures thereof are provided. The metal salt stabilizers minimize precipitation of the rhodium metal during recovery of the acetic acid product, particularly in flasher units in an acetic acid recovery scheme. Stability of the rhodium metal is achieved even when the acetic acid is produced in low water content reaction mixtures in the presence of an iodide salt co-promoter at a concentration that generated an iodide ion concentration of greater than about 3 wt. % of the reaction mixture. The stabilizing metal salts may be present in the reaction mixtures for the production of acetic acid at molar concentrations of metal to rhodium of about 0.1:1 to about 20:1. The stabilizing metal salts may be combined with other catalyst stabilizers as well as catalyst promoters.
Owner:CELANESE INT CORP
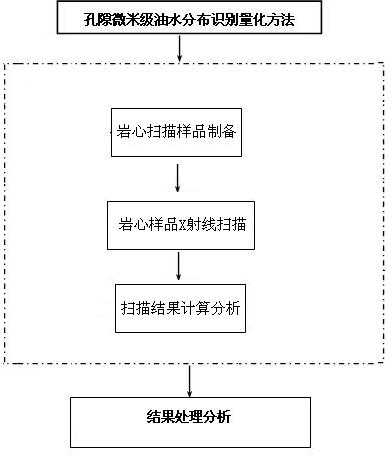
Pore micron-sized oil water distribution recognition and quantification method
InactiveCN102628354AEffective response distributionThe preparation process is simple and reliableSurveyFluid removalX-rayQuantification methods
The invention relates to a pore micron-sized oil water distribution recognition and quantification method. A flooding experiment scheme is firstly designed according to the research purpose, in different chemical flooding phases, iodide ions serving as a scanning standardizing reagent are added to a displacement chemical reagent, after each chemical flooding phase is over, a test sample is prepared, then an X ray scanning test is carried out, a scanning result is calculated and analyzed and automatically recorded by a computer, an X ray gray level distribution data graph obtained by scanning is transmitted to a data processing workstation unit of a microcosmic scanning system to be subjected to two-dimensional image reconstruction, gray level recognition and pore interior oil water distribution computing, so that core pore parameters and core pore interior oil water actual distribution image and quantification proportion are obtained. According to the invention, the sample preparation is simple and reliable, no damage is caused to the rock structure, the oil water distribution states in pores of a natural core and an artificial core in different chemical flooding phases are effectively reflected, the quantitative description is also provided, and the applicability of test results is good.
Owner:NORTHEAST GASOLINEEUM UNIV
Composition and method for controlling precipitation when acidizing wells
InactiveUS6225261B1Prevent precipitationEasy to cleanOther chemical processesCleaning apparatusDiketoneKetone
A composition and method for acidizing a formation in the presence of ferric ion, free sulfur and / or sulfides is disclosed. The composition employs a ferric ion reducing agent and a sulfide reactant separately or in combination. The ferric ion reducing agent is a thioalkyl acid. The sulfide reactant compounds include alpha-diketones and saturated and alpha-unsaturated cyclic ketones. In another aspect, the reducing activity of the thioalkyl acid is accelerated by the presence of a catalytic quantity of a compound or mixture of compounds which yield in acid solution cuprous ion, cupric ion or combinations thereof with iodide ion.
Owner:HALLIBURTON CO
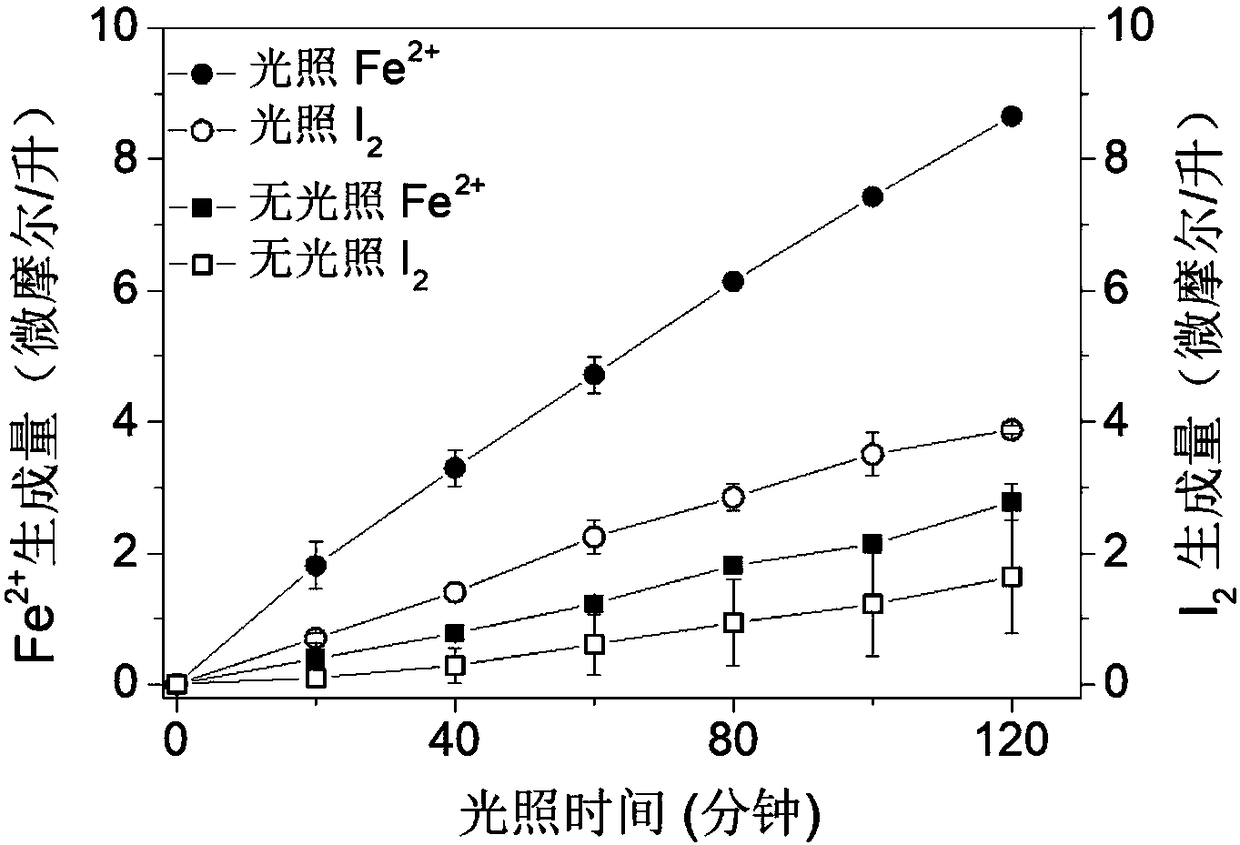
Method for recovering elemental sulfur through photoinduced catalytic disproportionation through sulfur dioxide absorption liquid
ActiveCN107720707AIncrease valueSolve pollutionSulfur preparation/purificationSulfurDisproportionation
The invention discloses a method for recovering elemental sulfur through photoinduced catalytic disproportionation through sulfur dioxide absorption liquid. According to the method, the sulfur dioxideabsorption liquid performs disproportionation reaction under the light illumination and iodide ion catalysis conditions to generate elemental sulfur precipitates. The method can be performed under the normal temperature and normal pressure conditions. Compared with a conventional high-temperature method, the method has the advantages that the energy consumption is low; the sulfur hardening is prevented; by using the method, the recovery of the elemental sulfur is easy; the flow process is short; the operation is simple; no secondary pollution exists; the industrial application is facilitated.
Owner:CENT SOUTH UNIV
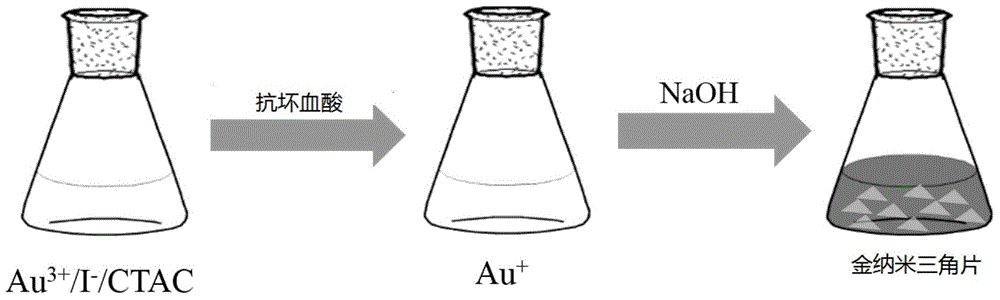
Method for quickly preparing high-yield gold triangular nanoprisms
The invention relates to a method for quickly preparing high-yield gold triangular nanoprisms. The method comprises the following steps of adding chloroauric acid solution and soluble iodide ion solution into a cationic surface active agent, namely quaternary ammonium chloride solution, taking ascorbic acid as a reducing agent, and adding aqueous alkali to regulate a pH value to induce the growth of the gold triangular nanoprisms with ascorbic acid as a reducing agent . According to the method for synthesizing the gold triangular nanoprisms, the gold triangular nanoprisms can be prepared in a very short time (about eight minutes), while for seed growth method-based synthesis of the gold triangular nanoprisms, synthesis steps are complicated, conditions are not easy to control, the repeatability is poor and three to four hours are required for completing the growth of the gold triangular nanoprisms. Therefore, the method has the advantages that the time for synthesizing the gold triangular nanoprisms is greatly shortened, the yield and the morphology uniformity of the obtained gold triangular nanoprisms are improved, the sizes of the gold triangular nanoprisms are adjustable, and a foundation is provided for widely applying the gold nanoprisms.
Owner:SUZHOU UNIV
Photothermographic recording material with in-situ and ex-situ photosensitive silver halide and a substantially light-insensitive organic salt
InactiveUS6274297B1Increase photosensitivityX-ray/infra-red processesPhotothermographic systemsSilver iodideSilver halide
Owner:AGFA HEALTHCARE NV
Gold plating liquid and gold plating method
InactiveUS20090038957A1Improve stabilitySimply and easily formedPhotography auxillary processesCoatingsPolyolDiethylene glycol
In a stable gold plating liquid having a low toxicity besides properties comparable to those of a cyan-type gold plating liquid, iodine and / or iodide ions, gold ions, and a polyalcohol having at least 4 carbon atoms are contained. The polyalcohol having at least 4 carbon atoms may be diethylene glycol or triethylene glycol. The content of the polyalcohol having at least 4 carbon atoms in the gold plating liquid is generally 10 to 90 percent by weight. The gold plating liquid may contain water.
Owner:MITSUBISHI CHEM CORP +1
Fluorescent molecular probe for detecting fluoride ions in aqueous solutions as well as synthesis method and application thereof
ActiveCN104418874AEasy to synthesizeMild reaction conditionsGroup 4/14 element organic compoundsFluorescence/phosphorescenceHydrogen SulfateSulfate radicals
The invention relates to a preparation method of a fluorescent molecular probe for detecting fluoride ions in aqueous solutions through fluorescence enhancement and an application of the fluorescent molecular probe to detecting fluoride ions. The fluorescent molecular probe is prepared by protecting 1,4-diethyl-1,2,3,4-tetrahydro-7-hydroxyquinoxaline-6-aldehyde taken as a raw material with silane and then condensing the raw material and malononitrile. The fluorescent molecular probe is simple and convenient to synthesize, and reaction conditions are mild. The fluorescent molecular probe has the specific characteristics that the probe molecule has stable optical properties and higher synthetic yield; the probe molecule has high sensitivity of detection of fluoride ions in the aqueous solutions and low lower limit of detection, and the limit of detection is 5.4mu M; the response range is 0-1mM and the detection range is wide; the probe molecule has good selectivity and has no responses to anions, such as chloride ions, bromide ions, iodide ions, tetrabutylammonium cyanide, nitrates radicals, hydrosulfate radicals, perchlorate radicals, acetate radicals, thiocyanate radicals, azide radicals, cysteine, bovine serum albumin, carbonate radicals, sulfate radicals and reduced glutathione; the fluorescent molecular probe has practical application values in the fields of biochemistry, environmental sciences and the like.
Owner:SUZHOU ROWLAND BIOTECH
Precious metal leaching agent and method of recovering precious metal in waste catalyst
ActiveCN110484745AImprove leaching efficiencyReduce use costProcess efficiency improvementPhosphonium saltBromine
The invention discloses a precious metal leaching agent and a method of recovering precious metal in a waste catalyst. The precious metal leaching agent comprises ionic liquid, iodoform and a solvent.The mass ratio of the ionic liquid to the iodoform is 30:1-5:1, and volume ratio of the solvent to the ionic liquid is 10:1-1:1. Negative ions of the ionic liquid are one or more kinds of chloride ions, bromine ions, iodide ions, thiocyanide ions and dual-nitrile amine root ions, and positive ions of the ionic liquid are one or more kinds of imidazole, pyrrolidine, quaternary ammonium salt and quaternary phosphonium salt. The precious metal leaching agent has the advantages of being economical, environmentally friendly and efficient. The invention provides the method of using the precious metal leaching agent to recover the precious metal from the waste catalyst. The method is simple in technological process and little in waste and byproduct emission, can effectively conduct harmless treatment on the waste catalyst and recover the precious metal in the waste catalyst, achieves the recycling of waste resources, reduces the environmental pollution and improves the social and economic benefit.
Owner:ZHEJIANG UNIV OF TECH
Iodine and iodide removal method
InactiveUS20080116417A1Sufficient solubilityReduce chain lengthHalogenated hydrocarbon separation/purificationHeat-exchange elementsMolecular sieveIon-exchange resin
A method for removing iodine and iodide ions from heat transfer compositions which contain a hydrofluoroalkene, an iodocarbon, and iodine and iodide ions. Iodine and iodide ions from such heat transfer compositions by contacting the composition with a molecular sieve, ion exchange resin, clay or alumina, metal impregnated with a metal which is capable of reacting with iodine and iodide ions.
Owner:HONEYWELL INT INC
Method of leaching copper sulfide ore with the use of iodine
ActiveUS8163063B2Efficient leachingEfficient executionSolvent extractionGold compoundsPregnant leach solutionChalcopyrite
An object of the present invention is to provide a method of efficiently leaching copper from a copper sulfide ore containing chalcopyrite or enargite as a main constituent under versatile conditions for actual operation.A method of leaching copper from a copper sulfide ore, characterized by comprising using, as a leaching solution, a sulfuric acid solution containing iodide ions and ferric (III) ions in an excessive amount relative to the iodide ions and leaching copper from a copper sulfide ore; or a method of leaching copper from a copper sulfide ore, characterized by comprising leaching copper from a copper sulfide ore with the use of a leaching solution further containing water-soluble ligands such as chloride ions that can stabilize ferric (III) ions in addition to the above components, is provided.
Owner:JX NIPPON MINING & METALS CORP
Preparation method of aerogel material capable of efficiently absorbing iodide ions and iodide steam
InactiveCN103111267AWide variety of sourcesLow costOther chemical processesDispersed particle separationNuclear fissionAmmonium hydroxide
The invention relates to a preparation method of an aerogel material capable of efficiently absorbing iodide ions and iodide steam, and relates to the preparation method of the aerogel materials. The preparation method of the aerogel material aims to solve the problem that at present, radioactive 129 I and 131 I generated in nuclear fission reaction are hard to catch and control, and easy to diffuse. The prepared material reacts with iodide ions and iodide steam, thereby mainly forming AgI and solidifying I2 monomers. The preparation method of the aerogel material comprises the following steps of 1. preparing natural macromolecular nano-fibril aqueous dispersion; 2. freezing and drying the natural macromolecular nano-fibril aqueous dispersion to form ultralight natural macromolecular nano-fibril porous materials, soaking the porous materials in a silver-ammonia solution, dispersing the porous materials by ultrasonic waves to be loaded with complex containing Ag+, and then freezing and drying the resultant again; 3. then soaking the resultant with thin alkali liquor again, and enabling the complex to react to form silver oxide to obtain nano-fibril loaded with the silver oxide; 4. and cleaning and drying the fibril to obtain the aerogel materials capable of efficiently absorbing iodide ions and iodide steam after being separated. The preparation method is applied in the aspects of nuclear industry, sewage treatment, electronic elements and the like.
Owner:NORTHEAST FORESTRY UNIVERSITY
Preparation method of gamma-copper iodide
The invention discloses a preparation method of gamma-copper iodide. The preparation method comprises the following steps: adding iodine into water so as to obtain an iodic aqueous solution and adding hydrazine hydrate into the iodic aqueous solution for mixed reaction to obtain an iodide ion solution, wherein the molar ratio of the iodine to the water is controlled at 1:(50-90) and the molar ratio of the hydrazine hydrate to the iodine is controlled at (4.1-4.5):1.0; and adding the iodide ion solution into a copper sulfate solution under the stirring effect, controlling the molar ratio of copper sulfate to iodine at (2.0-2.2):1.0, fully reacting, filtering and drying to obtain gamma-copper iodide. According to the preparation method, the iodine utilization rate is high, a product is less in possibility of being oxidized and decomposed, the production cost is low, and the process is simple.
Owner:GUIZHOU UNIV
Gold plating liquid and gold plating method
Disclosed is a gold plating liquid and a method for gold plating, wherein the gold plating liquid containing iodine and / or iodide ions, gold ions and a polyhydric alcohol having 4 or more carbon atoms. This gold plating liquid is stable and low in toxicity, while achieving performance comparable to cyan-type gold plating liquids. The polyhydric alcohol having 4 or more carbon atoms may be diethylene glycol or triethylene glycol. The amount of the polyhydric alcohol having 4 or more carbon atoms contained in the gold plating liquid is usually 10-90% by weight. The gold plating liquid may contain water.
Owner:MITSUBISHI CHEM CORP +1
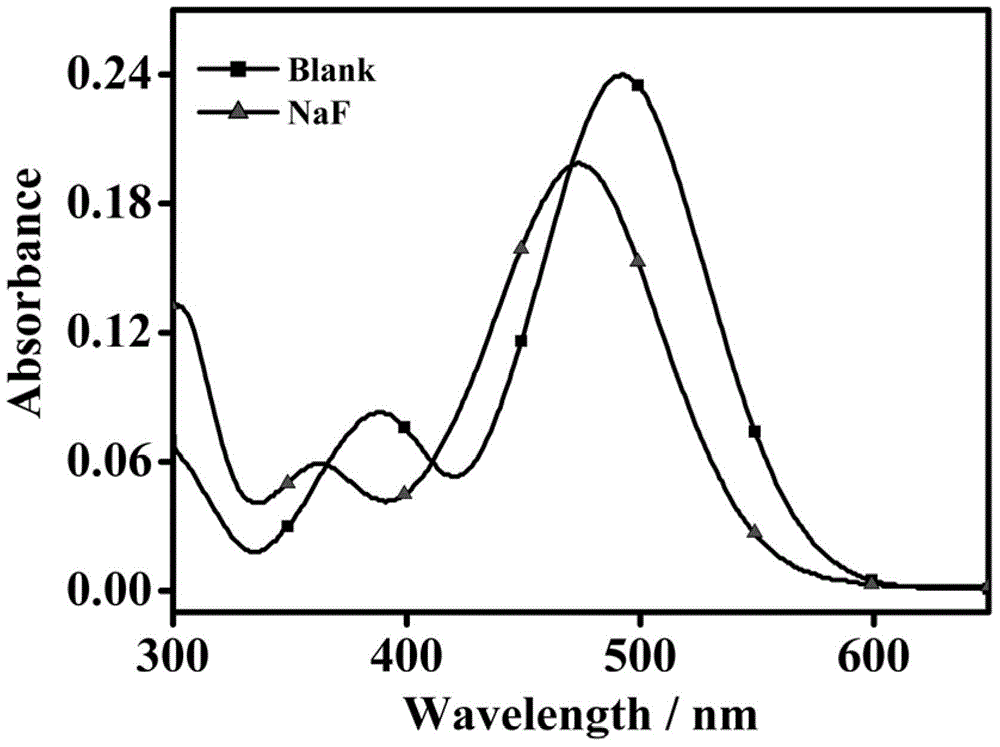
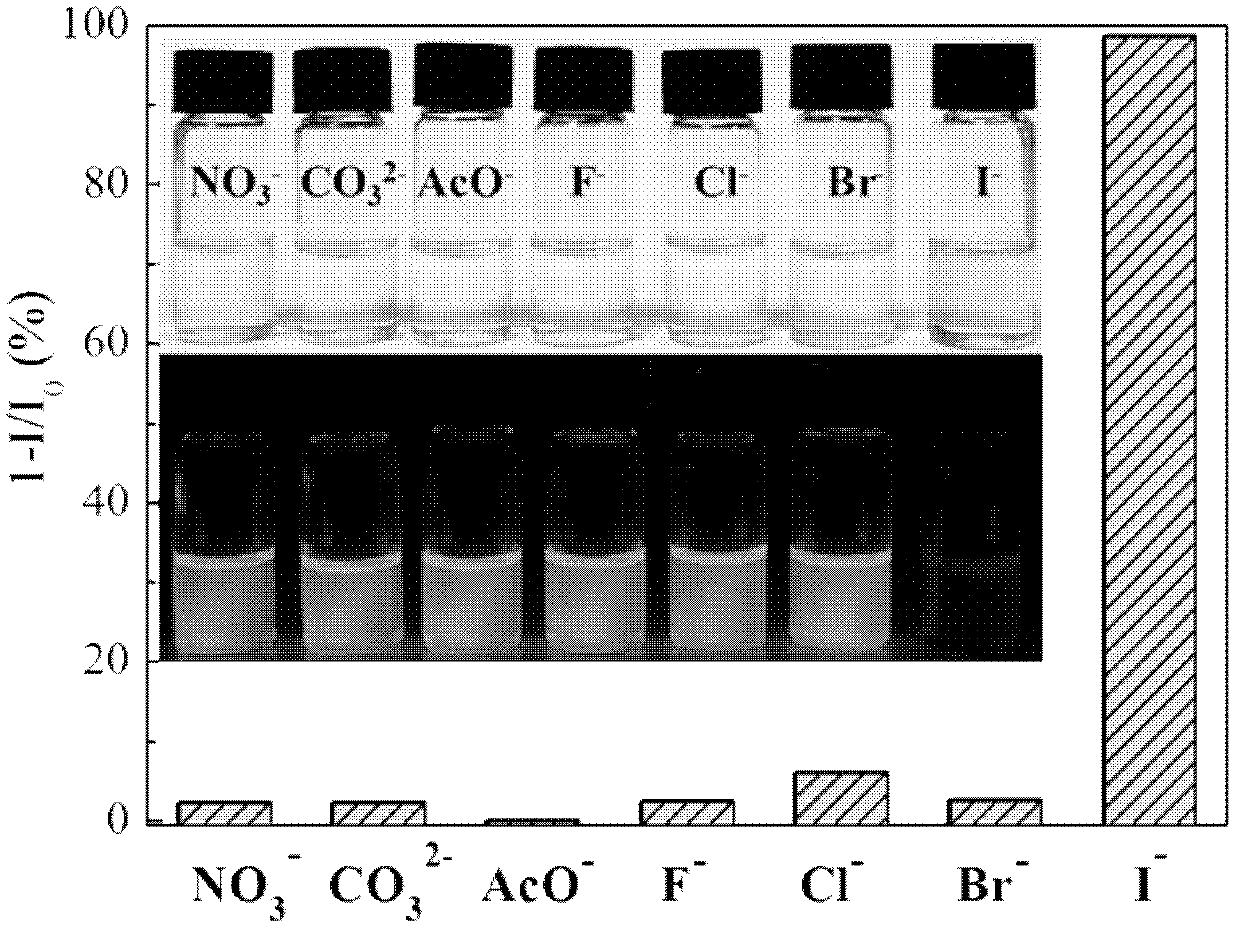
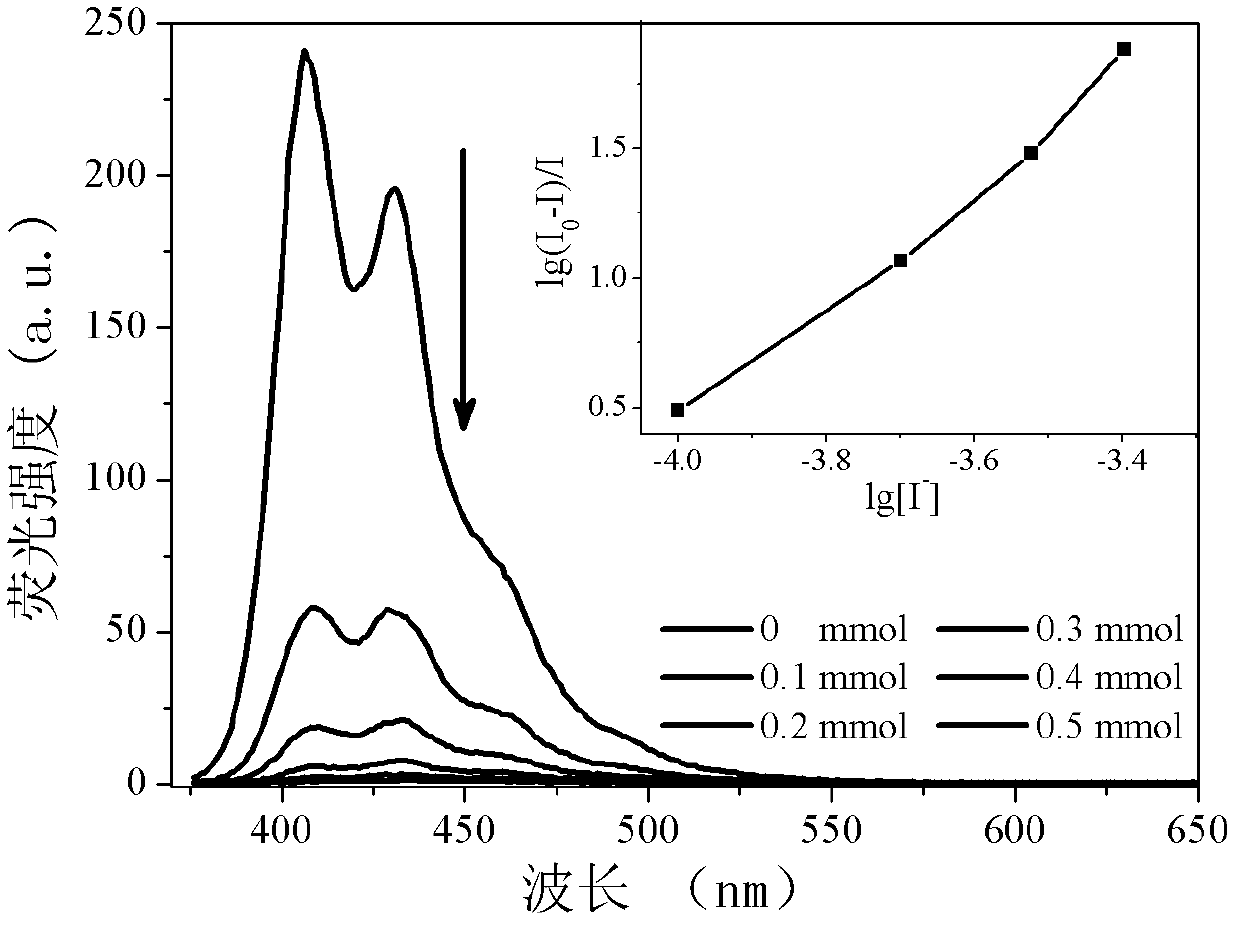
Iodide ion sensing material and its application in iodide ion fluorescence and chromogenic detection
InactiveCN102435588AOrganic chemistryMaterial analysis by observing effect on chemical indicatorCarbazoleSide chain
The invention belongs to the field of iodide ion (I-) sensing technology, and specifically relates to a sensing material with 2,7-position of fluorene as the main chain and flexible alkyl connected carbazole group as the side chain. The sensing material has advantages of inexpensive and easily-obtained raw materials, simple synthesis steps and excellent detection sensitivity and ion selectivity. In common negative ions, the material only has responses to I-. When the concentration of I- in the system is 0.3 mM, the fluorescence quenching of the solution is close to 100%, and there is basically no change with the addition of other negative ions. Apart from good fluorescence detection selectivity and sensitivity, the sensing material is more excellent in chromogenic detection. When the concentration of I- in the solution system reaches 0.1 mM, obvious color change (from colorless transparency to yellow) can be observed by naked eyes. The I- detectable concentration accounts for 1 / 100 ofcurrently reported I- chromogenic sensing material. Therefore, the iodide ion sensing material provided by the invention is a good sensing material and has a wide application prospect.
Owner:JILIN UNIV
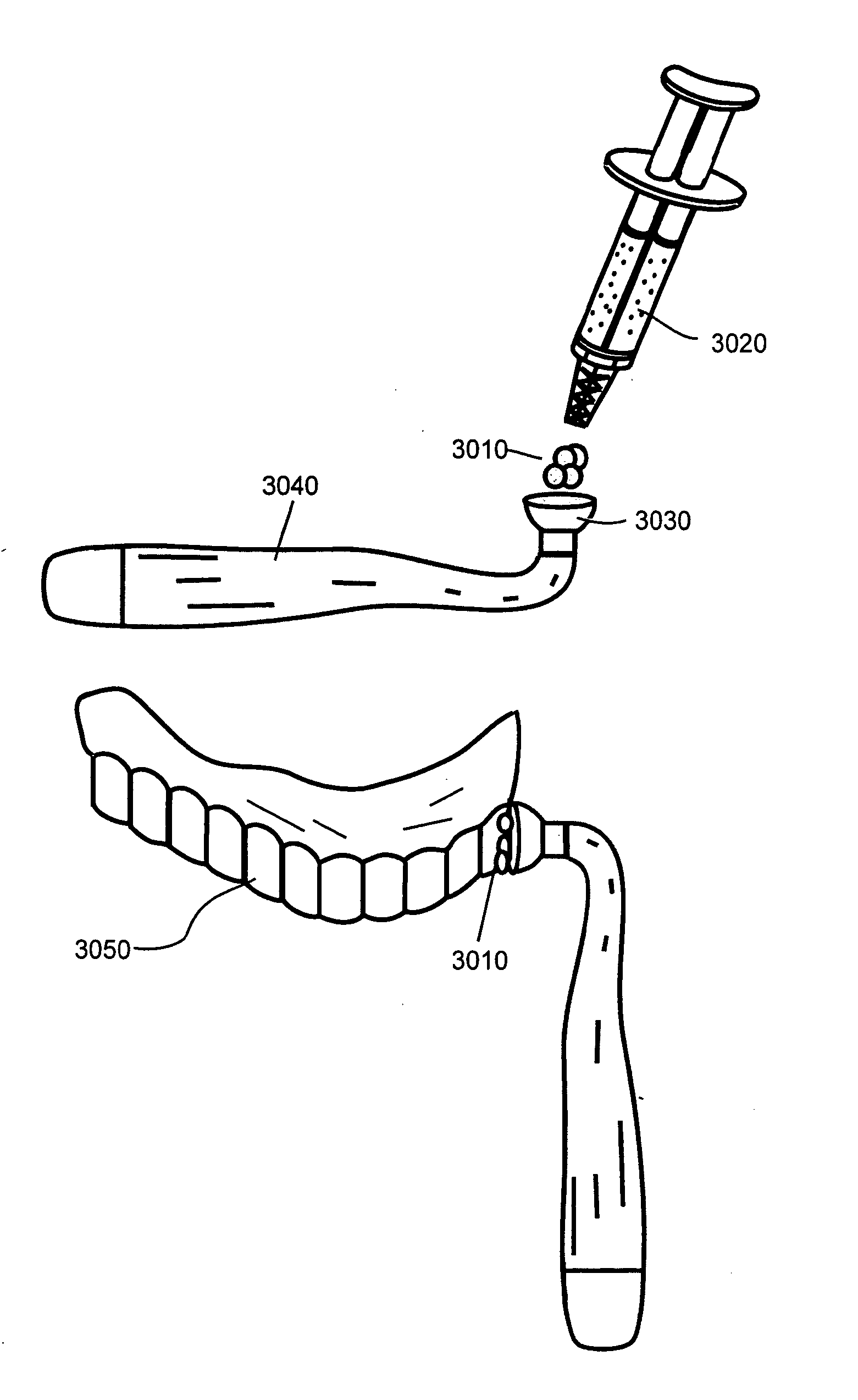
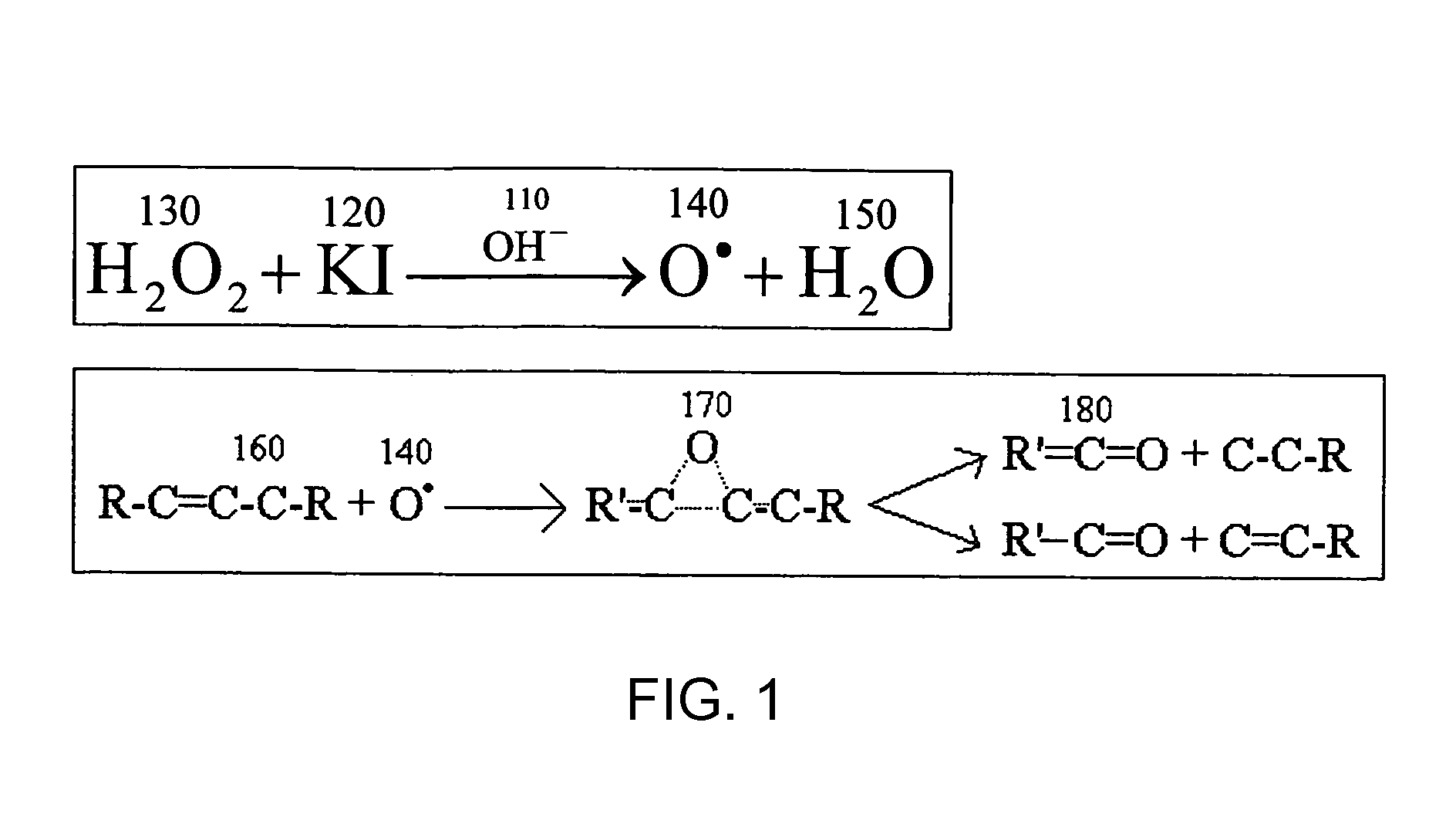
Bleaching Toothpastes and Methods for Making and Using Them
InactiveUS20080311057A1Improve efficiencyCosmetic preparationsToilet preparationsSolubilitySodium iodide
In toothpaste, the addition of the Iodide ion by way of iodide salts, such as sodium iodide and potassium iodide, to a peroxide such as hydrogen peroxide in a basic medium yields free radical oxygen and water; generating large amounts of heat and depleting the Hydrogen Peroxide in a matter of minutes. The free radical oxygen generated in this reaction can be utilized to oxidize organic molecules that produce offending stains on select items, including artificial teeth and other dental appliances. Once the free radical oxygen has oxidized the offending molecule the color is lost and the solubility changes allowing any loose fragments of the offending molecule to be washed away in the solvent. The iodide ion catalyzes the reaction allowing for precise control over the speed at which the stain is removed without the need for other expensive, cumbersome energy adding equipment such as lights, lasers, heat sources, etc.
Owner:CAO GROUP
Photoelectrochemical reaction tank and method of treating hydrogen sulfide waste gases and waste water by same
The invention relates to a method and a device for synchronizing hydrogen sulfide waste gas treatment and advanced waste water oxidation by taking sunlight as the driving force and recycling sulfur resources. A photoelectrocatalytic reactor applied comprises an anode chamber, a photo-anode, a cathode chamber, a cathode, an external circuit and an ion exchange membrane. The anode chamber is separated from the cathode chamber by the ion exchange membrane. The method and the device have the advantages that redox active substances (such as iodide ions and iron ions) are added into the anode chamber and the cathode chamber, and cavities formed through photocatalysis of the anode oxidize sulfur oxide ions indirectly to form high-purity recyclable elemental sulfur particles; peroxymonosulfate isactivated by ions (such as ferrous ions) with catalytic activity, generated through cathodic reduction, to generate free radicals, and pollutants in waste water are removed efficiently; the sunlight can be used as the only energy, and sulfur ions in recycled waste gases and organic pollutants in the waste water can be removed synchronously; the method and the device are applicable to treatment ofanaerobic digestion waste gases and sulfide-containing waste water / waste gases as well as water environment remediation.
Owner:SUZHOU INST FOR ADVANCED STUDY USTC
Method for recovering iodine
The invention discloses a method for recovering iodine, which is coupled with the electrochemical method to control the redox level, and recovers the inorganic iodine in liquid waste. A sodium hypochlorite solution is used for oxidizing and recovering organic iodine. The iodide ion content of the recovered liquid waste is about 0.3%. The iodine in the recovered liquid waste is gathered and recovered by using strong basicity anion exchange resin. The iodate ion is reduced to iodine by the method in one step. The complicated operations of reducing iodate ions into iodide ions firstly and then oxidizing into iodine are prevented. The usage amount of the oxidizing and reducing agents is reduced. Therefore, the cost is reduced, and the method for recovering iodine can bring high economic benefit to industrial production.
Owner:施一飞
Method for determining penetration depth of iodide ions in concrete
InactiveCN102590059ASimple processSurface/boundary effectPermeability/surface area analysisChloridePenetration depth
The invention discloses a method for determining the penetration depth of iodide ions in concrete, which comprises the following steps of: (101) splitting a test piece: splitting the test piece into two halves after a migration test is completed; (102) spraying a reagent: spraying a potassium iodate solution to the splitting face of concrete, and spraying a starch suspension; (103) acidifying the surface: spraying a weak acid solution when the splitting face of concrete is slightly dry after the step (102) is completed; and (104) measuring the penetration depth: measuring the penetration depth of iodide ions after the splitting face of concrete develops a color, wherein the penetration place of iodide ions displays brown. The test process of the determination method is not influenced by chloride ions inside concrete, the process is simple and quick, and durability design and life predication can be carried out on internally-doped concrete structures and structures subject to chloride ion corrosion.
Owner:SHENZHEN UNIV
Bi2O3-BiOI heterojunction visible-light response photocatalyst and preparation method thereof
InactiveCN104383950AImprove the performance of degrading organic matterEfficient separationPhysical/chemical process catalystsHeterojunctionElectron hole
The invention discloses a Bi2O3-BiOI heterojunction visible-light response photocatalyst and a preparation method thereof. The photocatalyst is of a film structure, and comprises a conductive substrate and a Bi2O3-BiOI heterojunction film positioned on the conductive substrate. The preparation method comprises the following steps: firstly, preparing a Bi2O3 film on the conductive substrate, then putting the Bi2O3 film in an iodide ion solution for ion exchange so as to obtain the Bi2O3-BiOI heterojunction visible-light response photocatalyst. Under the irradiation of sunlight, the photocatalyst disclosed by the invention shows relatively high visible light catalytic activity, and the photoelectrocatalysis degradation effect is relatively good; moreover, photoproduction electron-hole pair compounding is inhibited while transferring of electrons is promoted, and the Bi2O3-BiOI heterojunction visible-light response photocatalyst and the preparation method thereof can be used for degrading environment pollutants by photoelectrocatalysis, photoelectrocatalysis synthesis and hydrogen production through water photolysis.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Polyionic liquid base micropore quasi solid state electrolyte preparation method and application
InactiveCN101752090AReduce leakage and volatilizationPrevent leakageLight-sensitive devicesSolid-state devicesSolid state electrolyteSide chain
The invention provides a polyionic liquid base micropore quasi solid state electrolyte preparation method, belonging to the field of preparation process technology of solid state electrolyte; in the method, imidazole iodide ion liquid containing unsaturated double bond at side chain is prepared, and then radical polymerization is carried out to obtain polyionic liquid, and then the polyionic liquid is mixed with polyvinylidene fluoride, a polymer micropore film is prepared by a phase transition method, and then the polymer micropore film is soaked in the electrolyte to obtain the polyionic liquid base micropore quasi solid state electrolyte; the polyionic liquid base micropore quasi solid state electrolyte is applied to dye sensitization solar batteries, the problem that the battery is encapsulated difficultly is not only solved, and the long-time stability of the battery is improved, but also the charge transmission capacity in the quasi solid state electrolyte system is effectively improved and the performance of the quasi solid state dye sensitization solar battery is effectively improved.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
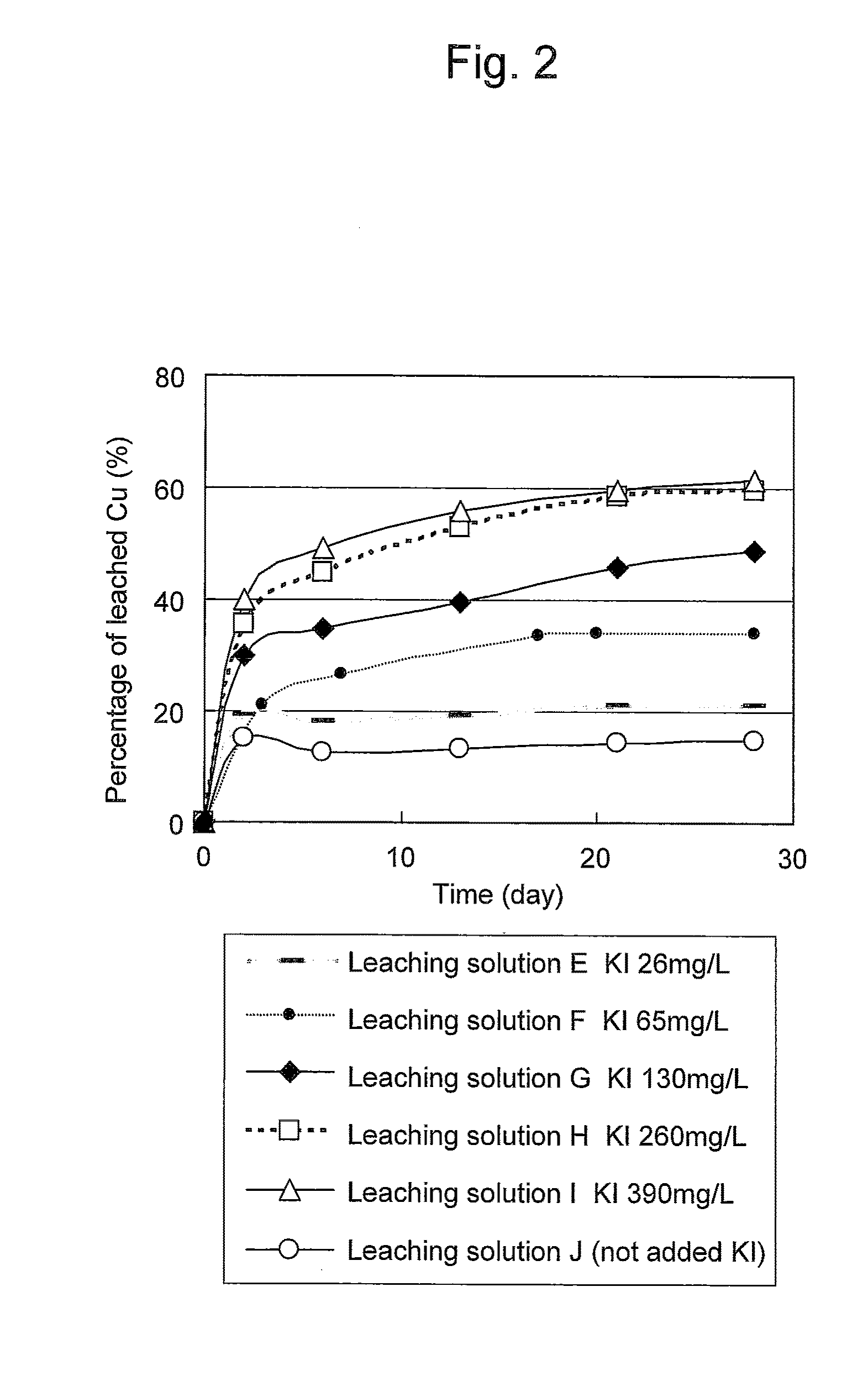
Method of heap or dump leaching of copper from copper sulfide ore
ActiveUS8287623B2Efficient leachingIncrease ratingsSolvent extractionGold compoundsDump leachingHeap leaching
Disclosed is a method of efficiently leaching copper not only from a readily-soluble copper ore but also a poorly-soluble copper sulfide ore partially containing or consisting of chalcopyrite and / or covellite by means of ore heap leaching under versatile conditions for actual operation. Also disclosed is a method of leaching copper from a copper sulfide ore, including leaching copper from an ore including a copper sulfide ore by heap or dump leaching with the use of a sulfuric acid solution containing ferric (III) ions and iodide ions at a total iodine concentration of 8 to 100 mg / L as a leaching solution.
Owner:JX NIPPON MINING & METALS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com