Dual-target parp/ezh2 inhibitor, preparation method and use
A dual-target, inhibitor technology, used in pharmaceutical formulations, medical preparations containing active ingredients, bulk chemical production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
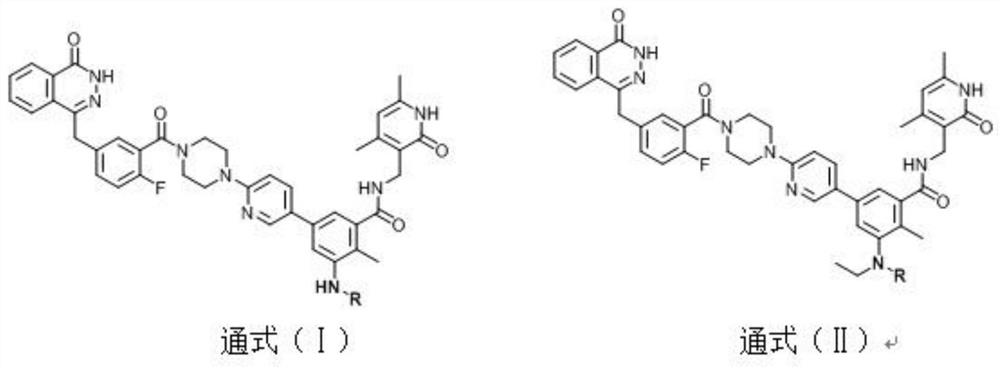
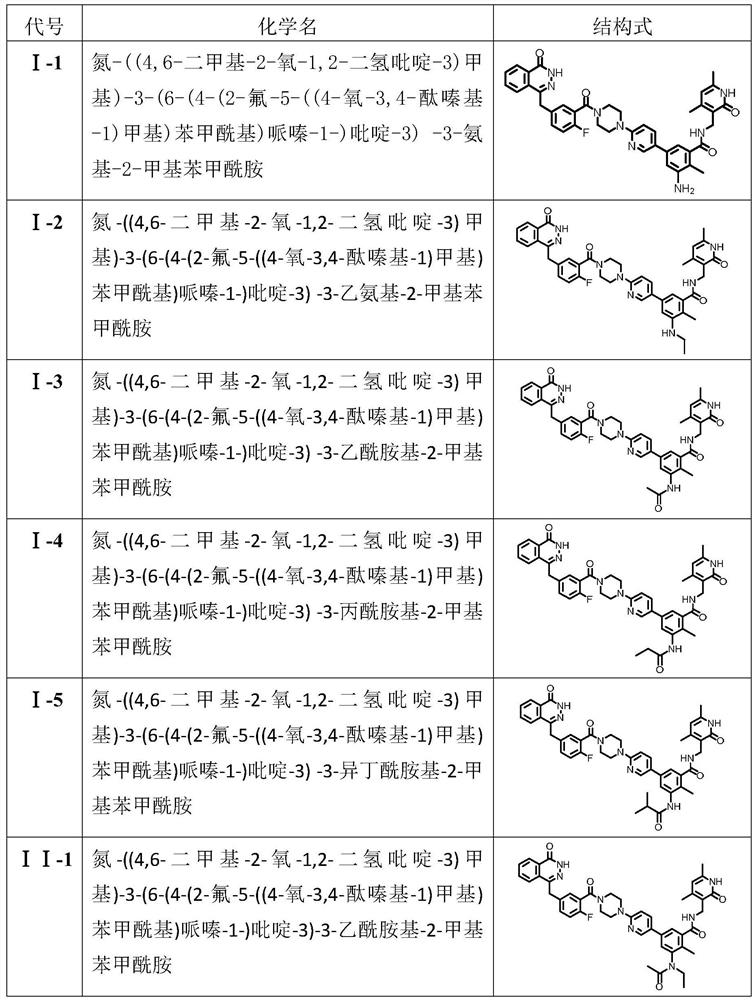
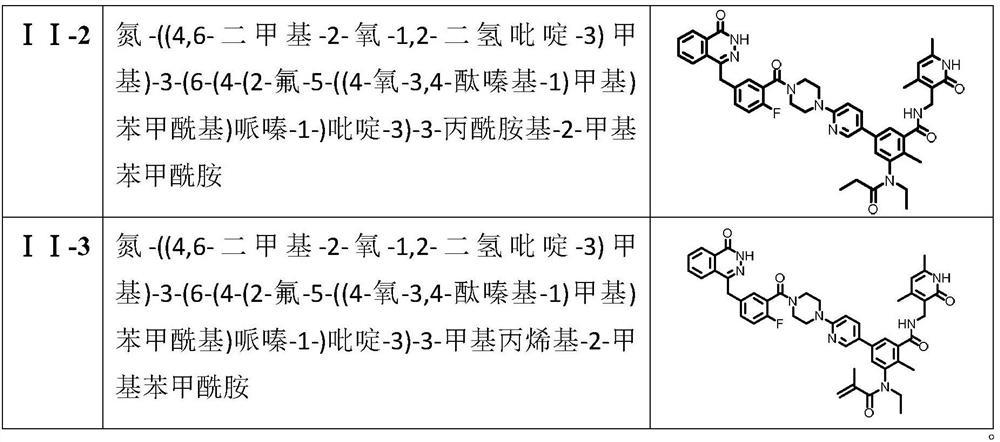
[0031] Compound (I-1): nitrogen-((4,6-dimethyl-2-oxo-1,2-dihydropyridine-3)methyl)-3-(6-(4-(2-fluoro- 5-((4-oxo-3,4-phthalazinyl-1)methyl)benzoyl)piperazine-1-)pyridine-3)-3-amino-2-methylbenzamide. Yield 52%.mp: 192-194°C; 1 H NMR (500MHz, DMSO-d 6 )δ(ppm): 12.61(s, 1H), 11.48(s, 1H), 8.33(d, J=2.5Hz, 1H), 8.27(d, J=7.5Hz, 1H), 8.02(t, J= 2.5Hz, 1H), 7.99(m, 1H), 7.90(t, J=7.5Hz, 1H), 7.85(t, J=7.5Hz, 1H), 7.74(m, 1H), 7.46-7.39(m, 2H), 7.26(t, J=9.0Hz, 1H), 6.90(d, J=9.0Hz, 1H), 6.87(d, J=2.5Hz, 1H), 6.67(d, J=2.5Hz, 1H, 5.86(s, 1H), 5.04(s, 2H), 4.35(s, 2H), 4.26(d, J=7.5Hz, 2H), 3.75-3.73(m, 2H), 3.67-3.65(m, 2H) , 3.52-3.49(m, 2H), 3.31-3.28(m, 2H), 2.20(s, 3H), 2.11(s, 3H), 2.02(s, 3H); 13 C NMR (125MHz, DMSO-d 6 )δ (ppm): 164.46, 163.98, 163.05, 159.40, 157.75, 156.42 (C, d, J C-F =202.5Hz), 149.39, 147.53, 145.00, 144.90, 138.80, 135.59, 134.86 (C, d, J C-C-C-C-F =2.5Hz), 133.53, 131.73 (C, d, J C-C-C-F =7.5Hz), 131.60, 129.10, 129.00 (C, d, J C-C-C-F =2.5Hz)...
example 2
[0033] Compound (I-2): nitrogen-((4,6-dimethyl-2-oxo-1,2-dihydropyridine-3)methyl)-3-(6-(4-(2-fluoro- 5-((4-oxo-3,4-phthalazinyl-1)methyl)benzoyl)piperazine-1-)pyridine-3)-3-ethylamino-2-methylbenzamide. Rate: 53%.mp: 192-194°C; 1 H NMR (500MHz, DMSO-d 6 )δ(ppm): 12.61(s, 1H), 11.47(s, 1H), 8.42(d, J=2.5Hz, 1H), 8.27(t, J=5.0Hz, 1H), 8.04(t, J= 5.0Hz, 1H), 7.98(d, J=5.0Hz, 1H), 7.91(t, J=7.5Hz, 1H), 7.86(t, J=7.5Hz, 1H), 7.61(s, 1H), 7.46 -7.40(m, 2H), 7.26(t, J=9.0Hz, 1H), 6.90(d, J=9.0Hz, 1H), 6.68(d, J=9.0Hz, 2H), 5.86(s, 1H) , 4.35(s, 2H), 4.27(d, J=2.5Hz, 2H), 3.75-3.73(m, 2H), 3.67-3.65(m, 2H), 3.52-3.49(m, 2H), 3.31-3.28 (m, 2H), 2.80(d, J=5.0Hz, 3H), 2.69(s, 1H), 2.20(s, 3H), 2.10(s, 3H), 2.03(s, 3H); 13 CNMR (125MHz, DMSO-d 6 )δ (ppm): 169.51, 163.98, 163.03, 159.40, 157.74, 156.42 (C, d, J C-F =202.5Hz), 149.39, 147.24, 145.23, 144.91, 142.67, 138.63, 135.90, 135.13, 134.86 (C, d, J C-C-C-C-F =2.5Hz), 133.54, 131.73 (C, d, J C-C-C-F =7.5Hz), 131.62, 129.10, ...
example 3
[0035] Compound (I-3): nitrogen-((4,6-dimethyl-2-oxo-1,2-dihydropyridine-3)methyl)-3-(6-(4-(2-fluoro- 5-((4-Oxo-3,4-phthalazinyl-1)methyl)benzoyl)piperazine-1-)pyridine-3)-3-acetamido-2-methylbenzamide. Yield: 50%.mp: 220-222°C; 1 H NMR (500MHz, DMSO-d 6 )δ(ppm): 12.61(s, 1H), 11.49(s, 1H), 9.44(s, 1H), 8.41(d, J=2.5Hz, 1H), 8.28(t, J=5.0Hz, 1H) , 7.98(d, J=5.0Hz, 1H), 7.91(t, J=7.5Hz, 1H), 7.85(t, J=7.5Hz, 2H), 7.61(s, 1H), 7.46-7.40(m, 2H), 7.29(s, 1H), 7.25(t, J=9.0Hz, 1H), 6.92(d, J=9.0Hz, 1H), 5.87(s, 1H), 4.35(s, 2H), 4.31( d, J=2.5Hz, 2H), 3.75-3.73(m, 2H), 3.67-3.65(m, 2H), 3.52-3.49(m, 2H), 3.31-3.28(m, 2H), 2.20(s, 3H), 2.16(s, 3H), 2.11(s, 3H), 2.08(s, 3H); 13 C NMR (125MHz, DMSO-d 6 )δ (ppm): 168.59, 168.44, 163.99, 163.02, 159.42, 157.92, 156.42 (C, d, J C-F =202.5Hz), 149.58, 145.30, 144.92, 142.78, 138.94, 137.51, 135.78, 134.86 (C, d, J C-C-C-C-F =2.5Hz), 134.43, 133.55, 131.73 (C, d, J C-C-C-F =7.5Hz), 131.62, 129.11, 129.00 (C, d, J C-C-C-F =2.5Hz),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com