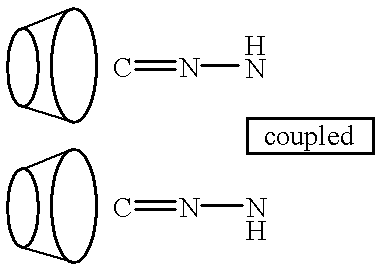
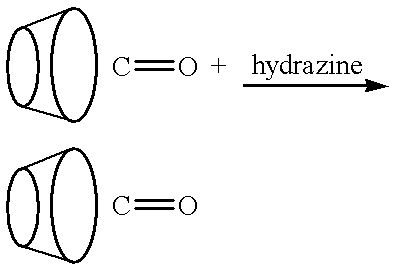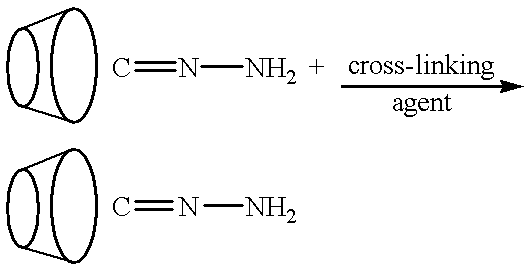Cyclodextrin polymer compositions for use as drug carriers
a technology of cyclodextrin and polymer composition, which is applied in the field of cyclodextrin polymer composition for use as drug carriers, can solve the problems of limited disclosure or suggestion of any cyclodextrin, the pharmacokinetics of many antiviral and anti-cancer drugs and other active agents that penetrate cells are not easily controlled, and the drug carrying capacity is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation ii
Cyclodextrin Polymer Carrier with Completely Entrapped 2AA
[0215] The purpose is to prepare a water-soluble (or colloidal) cyclodextrin polymer carrier with completely entrapped 2-aminoanthracene (2AA). The procedure was to combine 0.5 ml of 4.4% beta cyclodextrin in water, with 0.02 ml of solution containing 80% 1,4 butanediol diglycidyl ether (BDE), 10% of 0.1 M guest molecule, 2-aminoanthracene in dimethylformamide, and 2.4% 2 N NaOH while mixing vigorously and incubating at 60.degree. C.
[0216] After about 1 hour, 0.2 ml of 0.01 M K.sub.2HPO.sub.4 (K2 buffer, pH 8.6), and 0.02 ml more of BDE was added and mixed to continue the crosslinking. After about one half hour more, the mixture was mixed with 0.1 ml of 1M lysine for about 2.5 hours more. The preparation was then centrifuged for 8 minutes at 2500 rpm and 0.55 ml of supernatant was fractionated on a column of Sephadex.RTM. G-25 (14.times.0.8 cm) equilibrated with K2 buffer.
[0217] The 0.5 ml fractions were then collected and ex...
preparation xiv
Cyclodextrin Catalyst Agent
[0338] A cyclodextrin catalyst agent is a new invention defined herein as a cyclodextrin derivative of an individual cyclodextrin, or dimer, trimer or polymer, wherein the CD derivative host functions as an "artificial enzyme", and certain guest molecules function as chemical substrates. When the chemical substrate comes in contact with the cyclodextrin catalyst agent under appropriate conditions, it is modified to produce a product that is inhibitory or toxic to certain cells, microbes or parasites. In one embodiment, the CD catalyst agent is coupled to a biorecognition molecule for targeting specific infected cells, cancer cells, tissues or disease organisms.
[0339] With suitable derivatization, the CD catalyst agents can be synthesized to bind and modify prodrugs into active drugs and modify other specific substrates. The CD catalyst agent can also catalyze specific reactions such as generate free radicals, including singlet or triplet oxygen that direct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| spacer length | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com