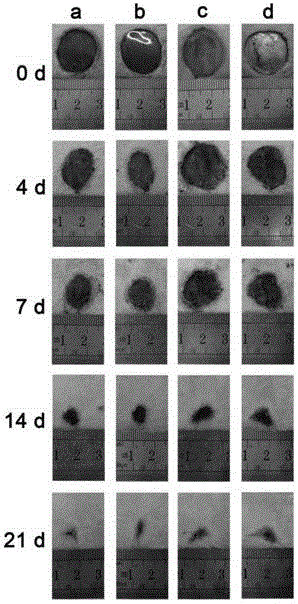
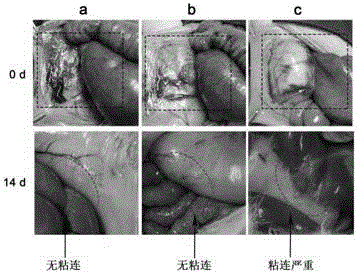
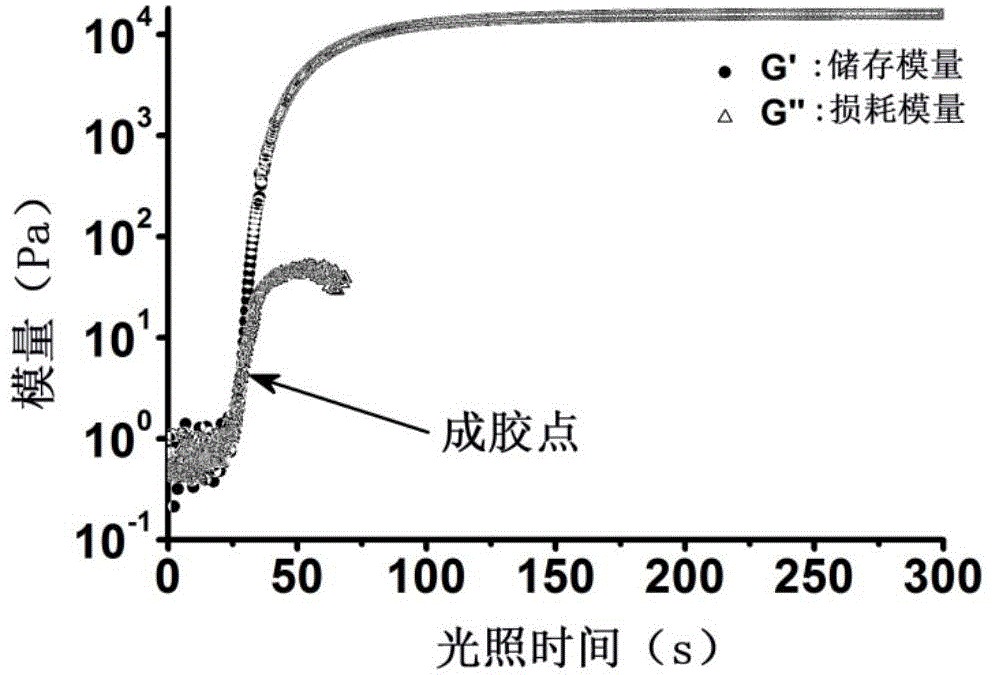
Non-radical photochemical crosslinked hydrogel material preparation method, product and application
A hydrogel and free radical technology, applied in biochemical equipment and methods, cosmetic preparations, medical preparations of non-active ingredients, etc., can solve problems such as toxic side effects, sensitivity, and difficulty in in-situ gelation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Synthesis of o-nitrobenzyl-modified hyaluronic acid derivatives (HA-NB)
[0065]
[0066] (1) Synthesis of Compound 1: According to references Pauloehrl, T.; Delaittre, G.; Bruns, M.; Meiβler, M.; H.G.; Bastmeyer, M.; Barner-Kowollik, C.Angew.Chem.Int.Ed.
[0067] (2) Synthesis of compound 2: Dissolve compound 1 (1g, 3.3mmol) and ethylenediamine (1.1mL) in methanol (50mL), reflux for overnight reaction, rotary evaporation under reduced pressure, and dissolve the crude product in methanol , and reprecipitated from ethyl acetate. After several times of dissolution-reprecipitation, filtration and vacuum drying, the pure compound 2 (0.93 g, yield 85%) was obtained.
[0068] (3) Synthesis of HA-NB: Dissolve hyaluronic acid HA (400mg) in 50mL distilled water until completely dissolved, add hydroxybenzotriazole (HOBt, 153mg), and then dissolve compound 2 (224mg, 0.69mmol) and 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC-HCl, 200mg) were add...
Embodiment 2
[0069] Example 2: Synthesis of dextran derivatives (dextran-NB) modified by o-nitrobenzyl groups
[0070]
[0071] (1) Synthesis of compound 3: according to references Pauloehrl, T.; Delaittre, G.; Bruns, M.; Meiβler, M.; H.G.; Bastmeyer, M.; Barner-Kowollik, C.Angew.Chem.Int.Ed.
[0072] (2) Synthesis of dextran-NB: Weigh 1g of dry Dextran and dissolve it in dry DMSO solution, compound 3 (0.23g, 0.62mmol), EDC-HCl (0.76g, 3.96mmol) and DPTS (0.12g ) were sequentially added to the above-mentioned dextran solution, and stirred and reacted for 48h at room temperature. After the reaction, pour the solution into cold ethanol for re-precipitation, purify through three dissolution-re-precipitation processes, and obtain pure dextran-NB (0.8g) after vacuum drying. According to its H NMR spectrum picture , it can be calculated that the degree of modification of the o-nitrobenzyl group is about 10%.
Embodiment 3
[0073] Embodiment three: the synthesis of the chitosan derivative (chitosan-NB) of o-nitrobenzyl modification
[0074]
[0075] (1) Synthesis of small bromo-o-nitrobenzyl molecules:
[0076]
[0077] (2) Synthesis of Compound 4: Weigh vanillin 3-methoxy 4-hydroxybenzaldehyde (0.76g, 4.9mmol), K 2 CO 3 (1.37g, 9.9mmol) and dibromoethane (1.28g, 6.9mmol) were co-dissolved in a dry DMF solution and reacted at 80°C for about 1h. After the reaction was completed, the reaction solution was poured into ice water to precipitate, filtered and washed. The crude product was purified by column chromatography (petroleum ether:ethyl acetate=8:2) to obtain pure compound 4 (1.1 g, 80%).
[0078] (3) Synthesis of Compound 5: Compound 4 (2.00 g, 7.32 mmol) was dissolved in 15 mL of ice-cold concentrated H 2 SO 4 Medium to completely dissolved. Guanidine nitrate (0.9 g, 7.37 mmol) was slowly added to the above solution, and reacted at room temperature for about 30 min. After the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com