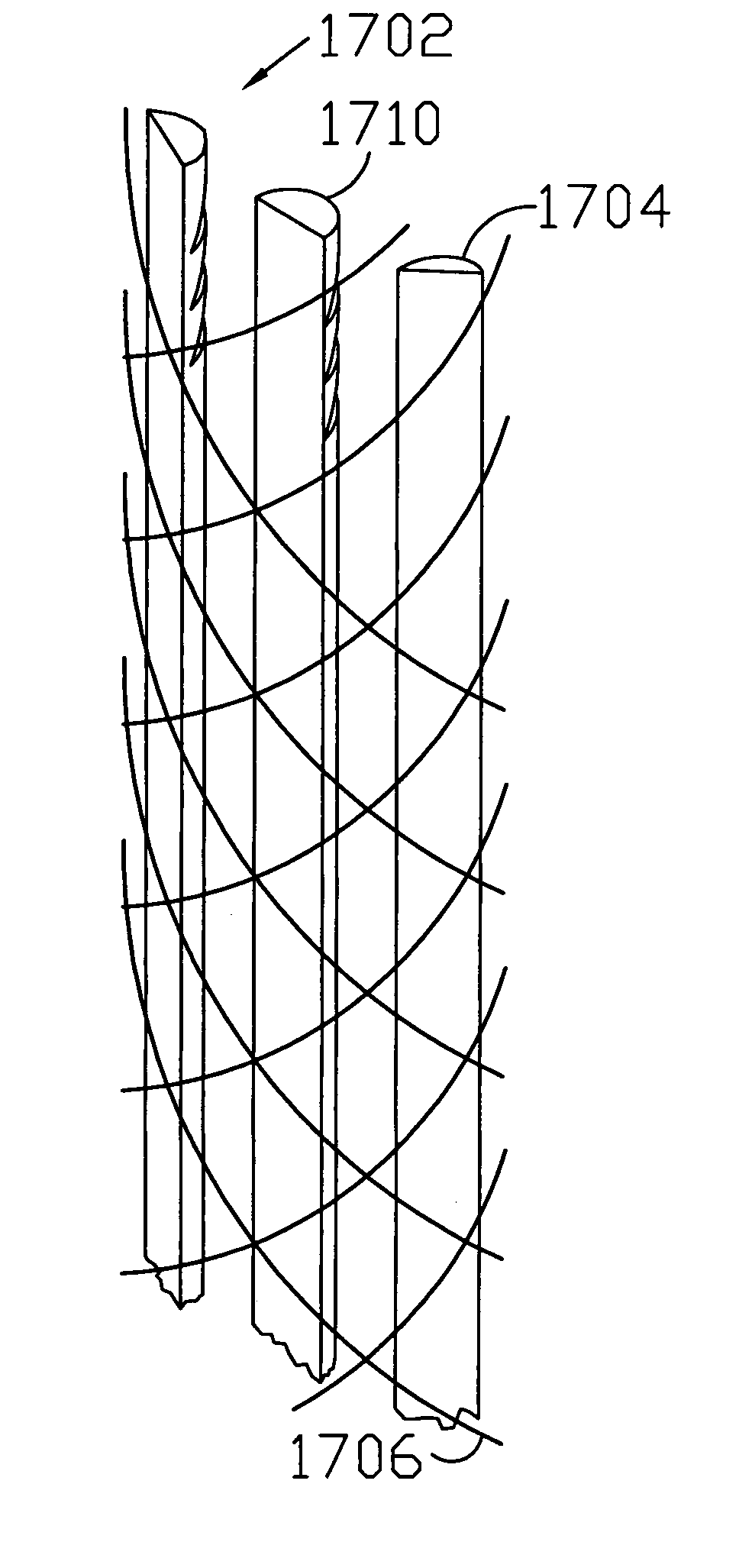Patents
Literature
139 results about "Seroma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A pocket formed by the accumulation of serous fluid after surgery.
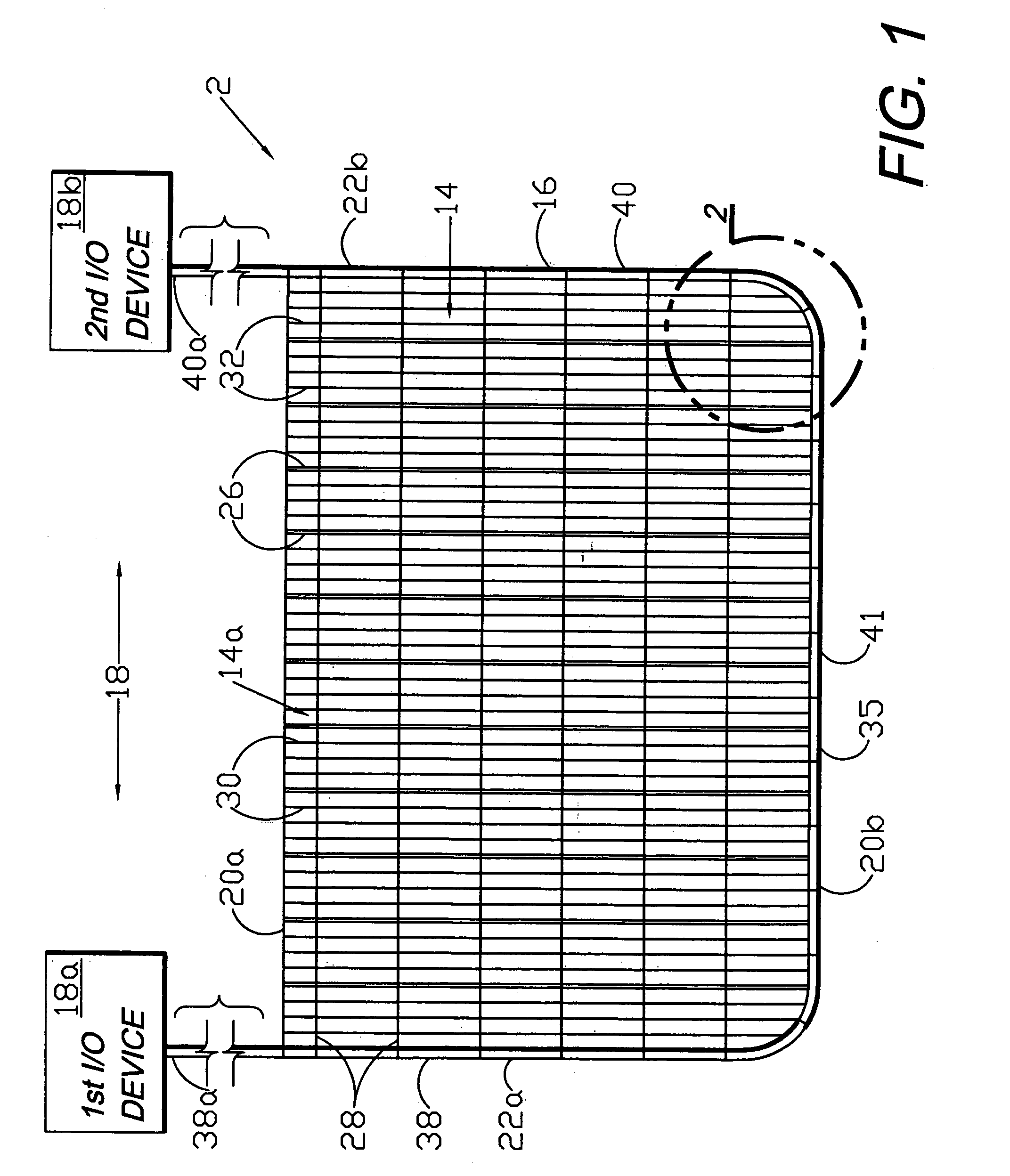
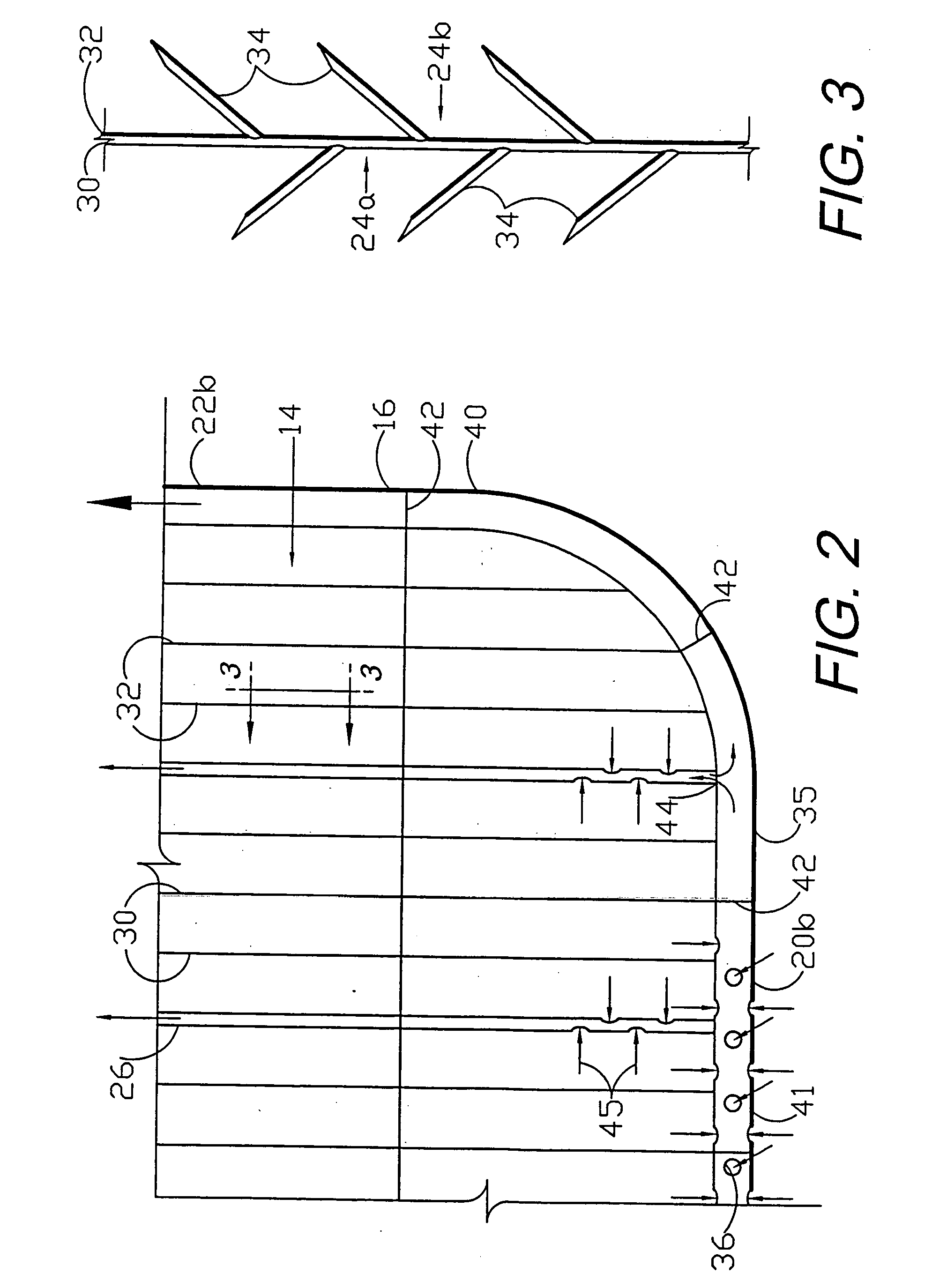
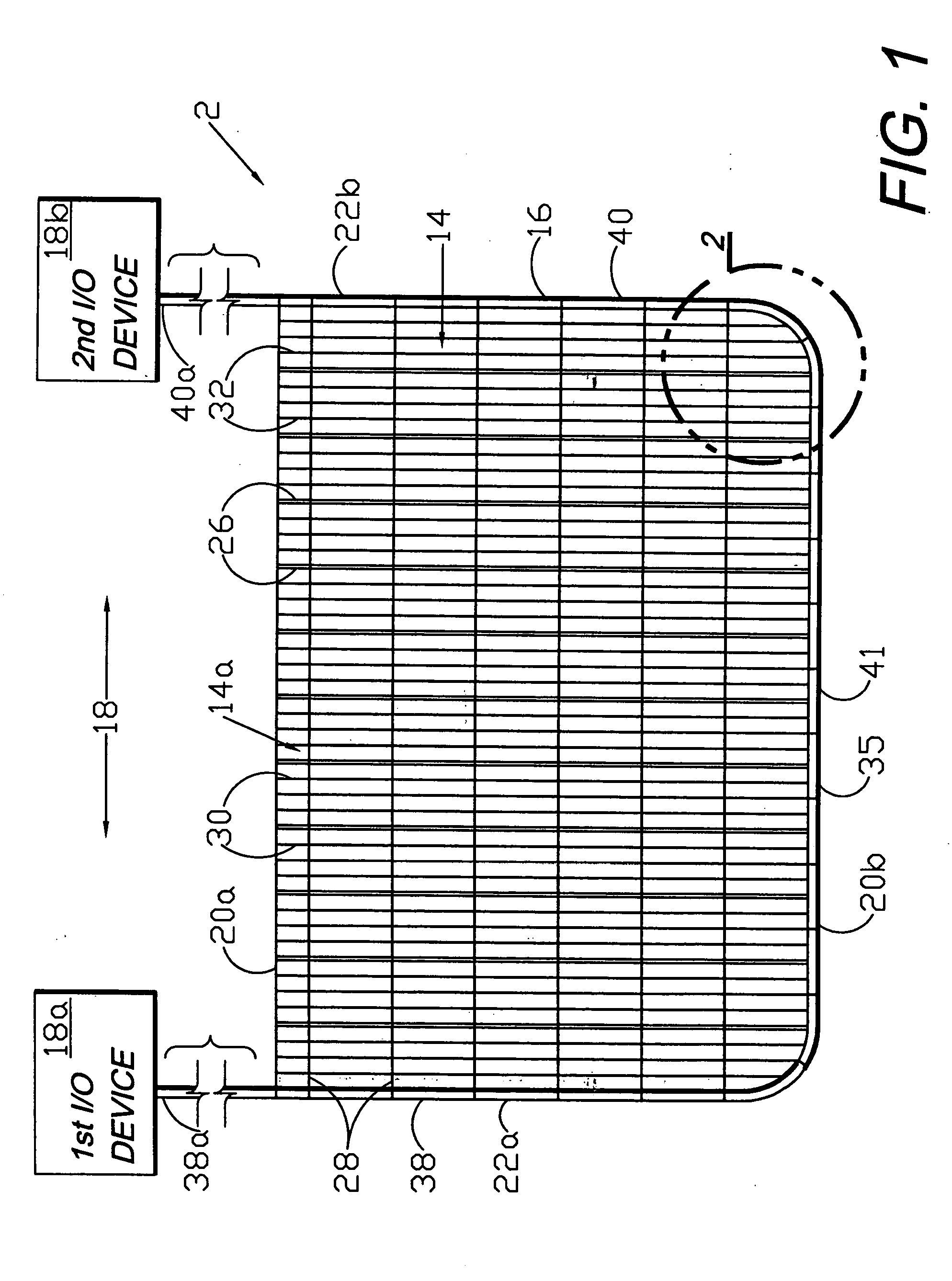
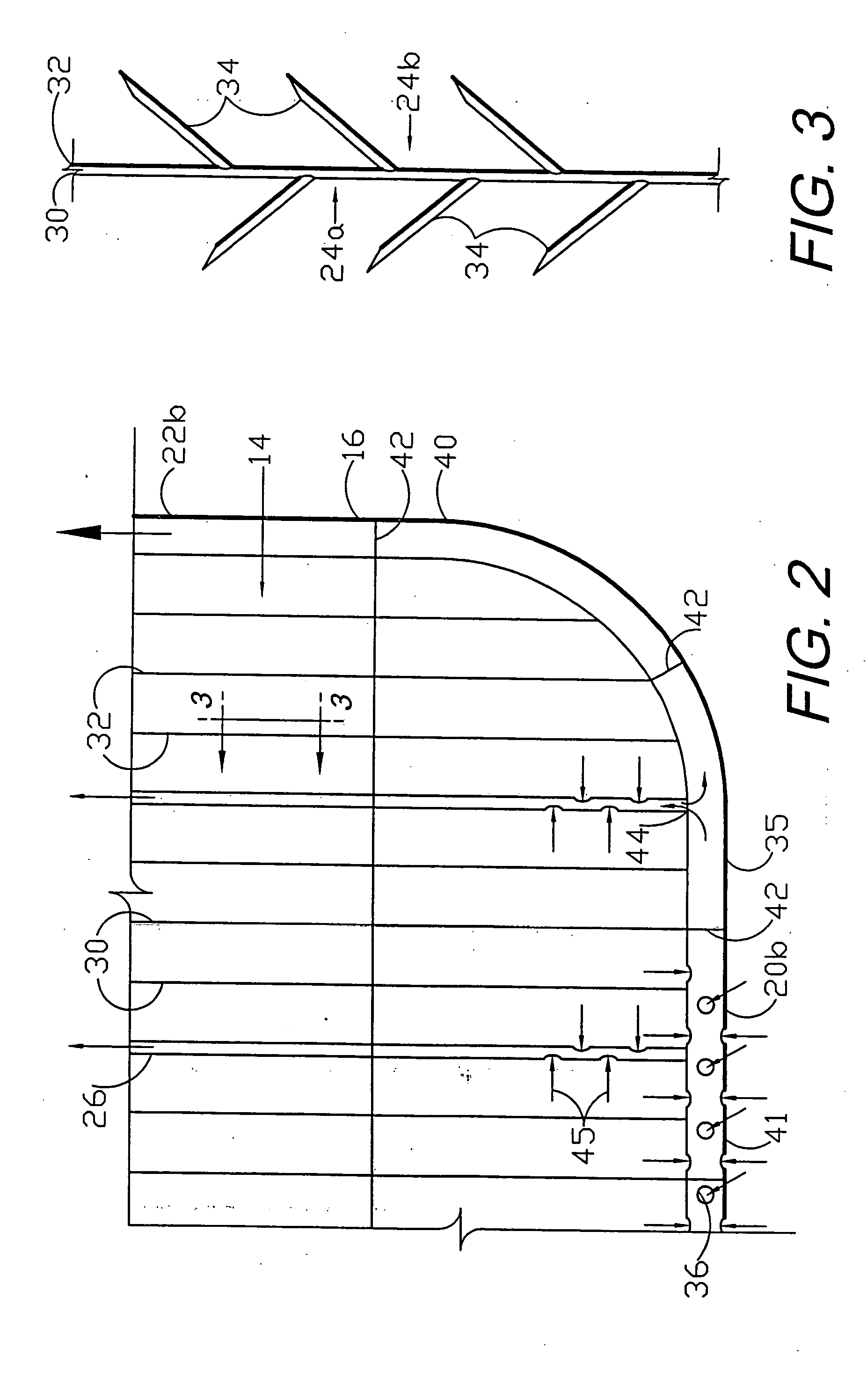
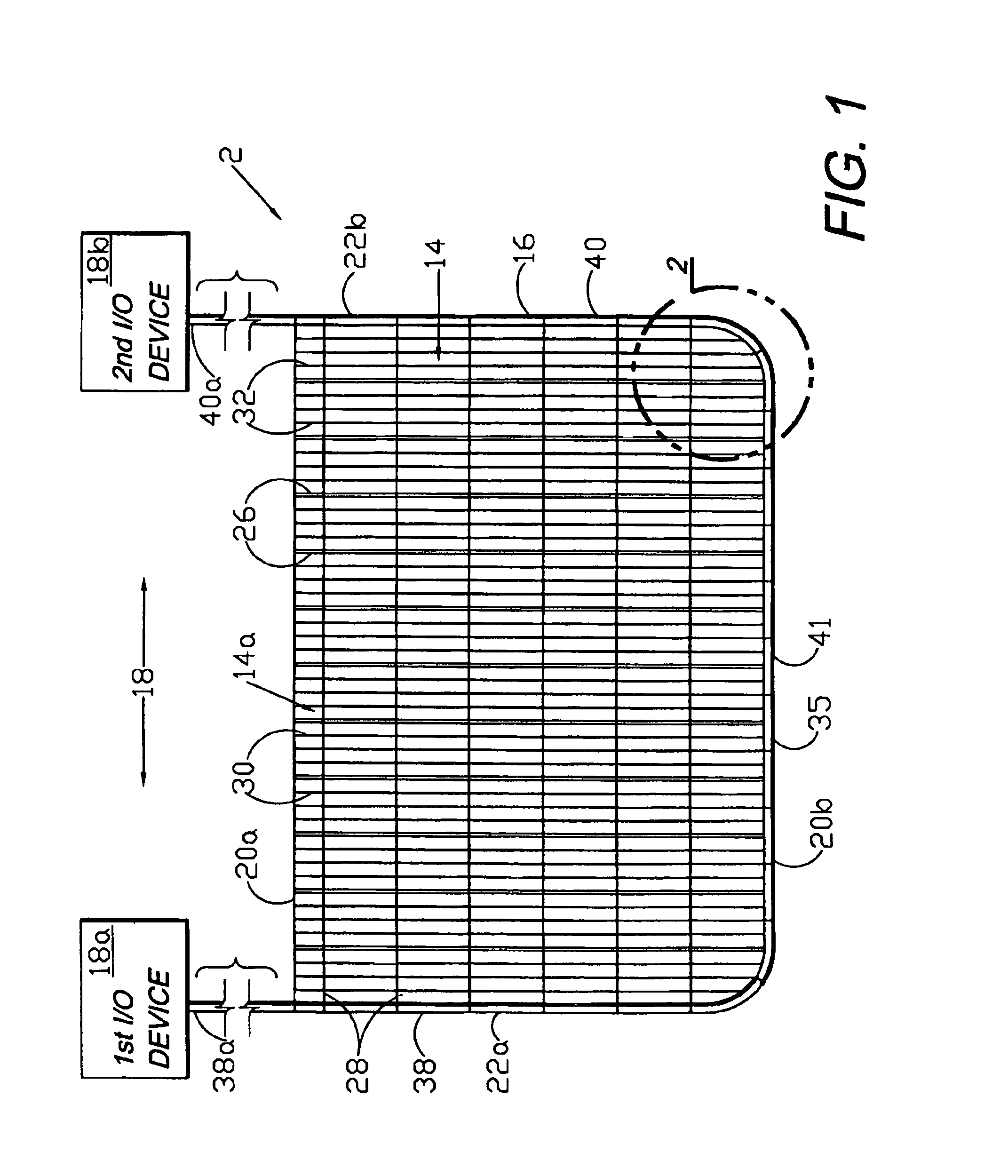
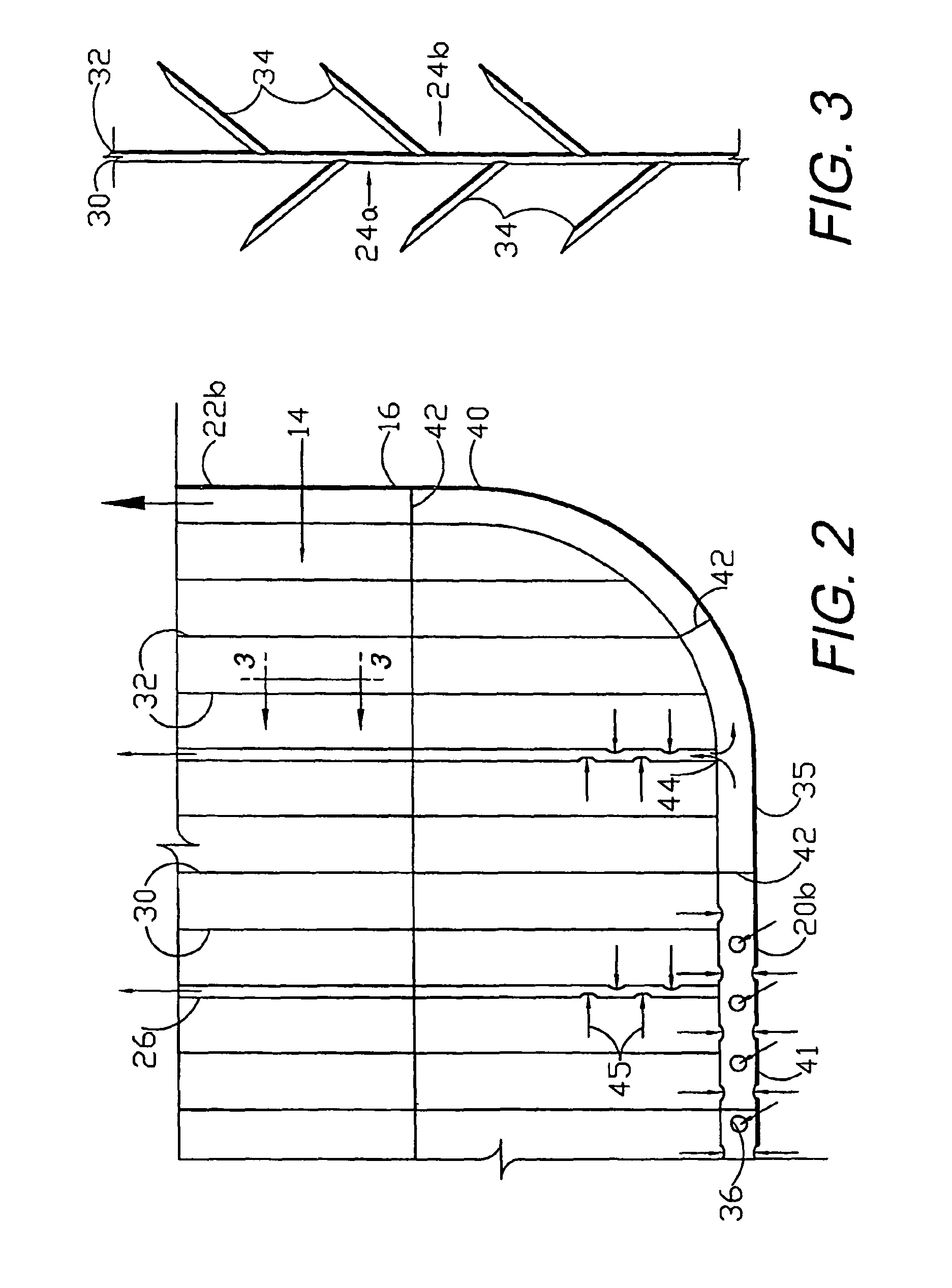
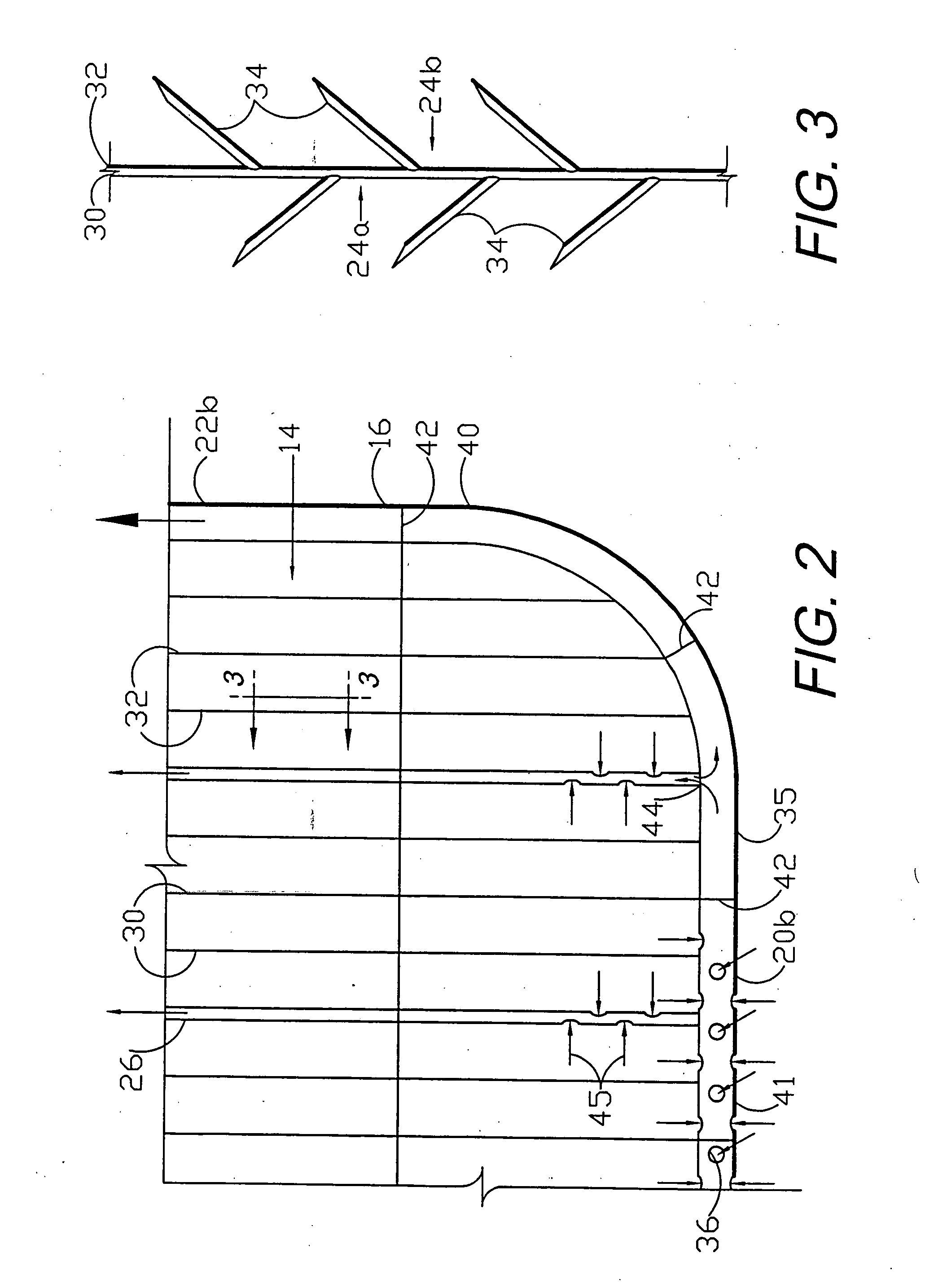
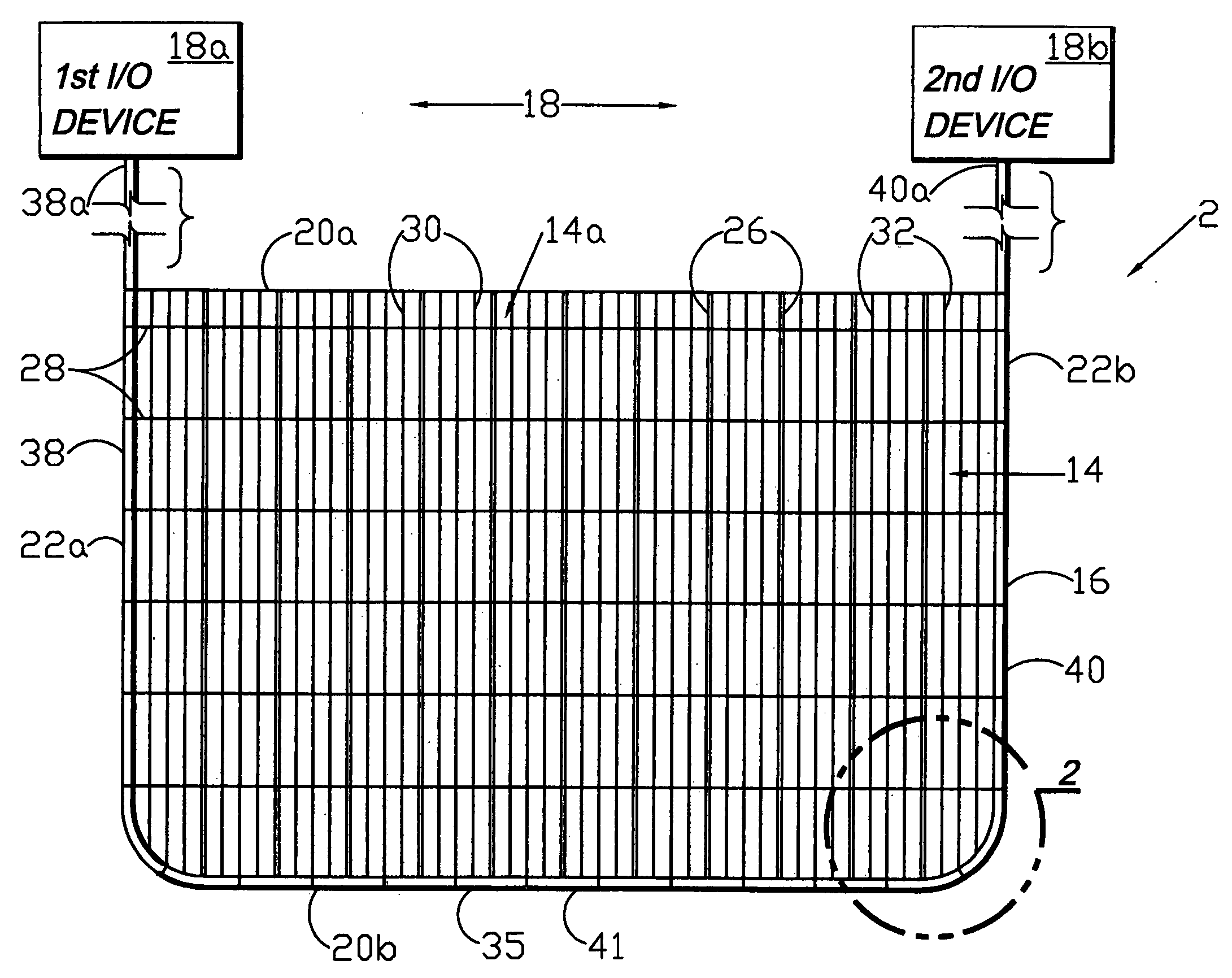
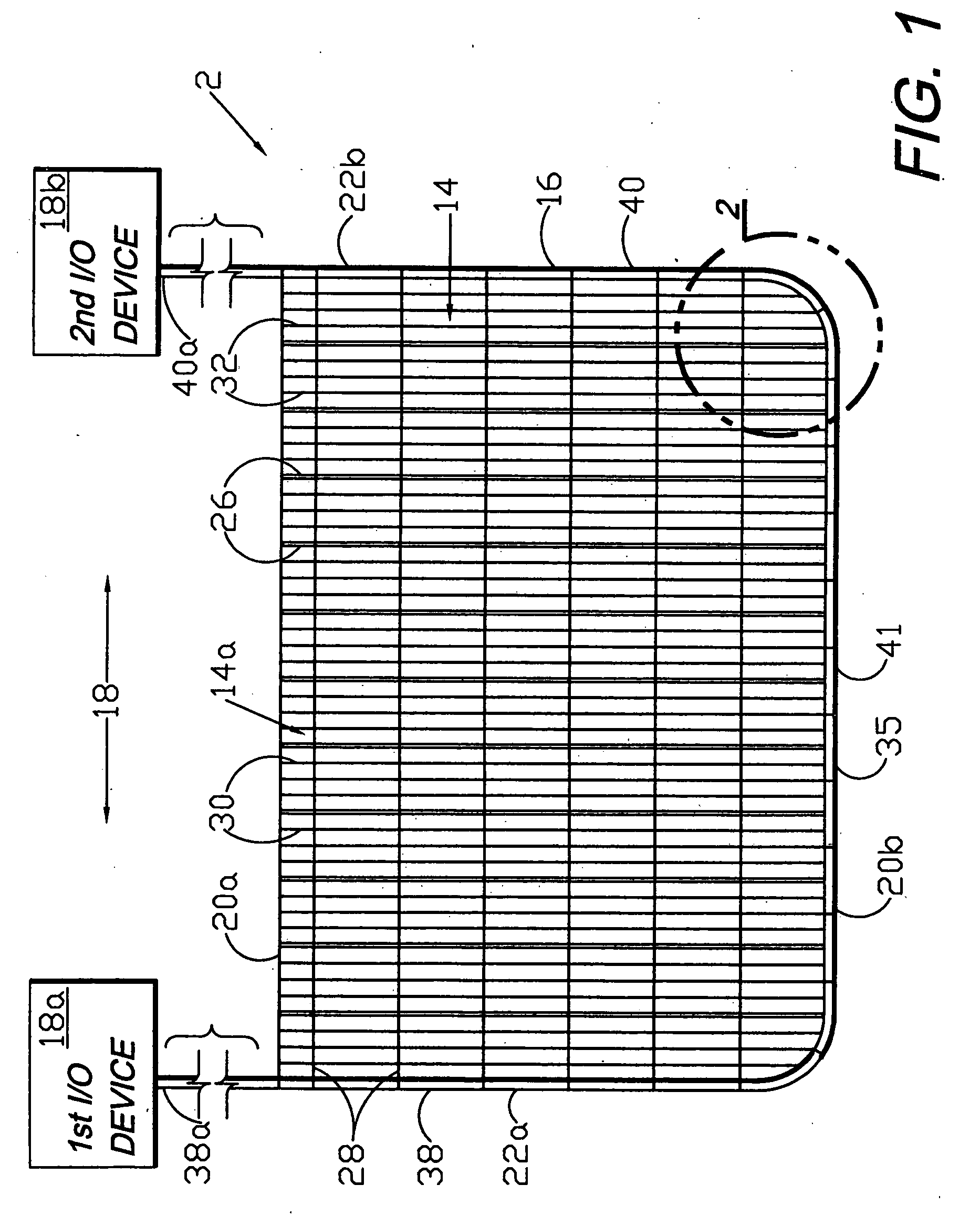
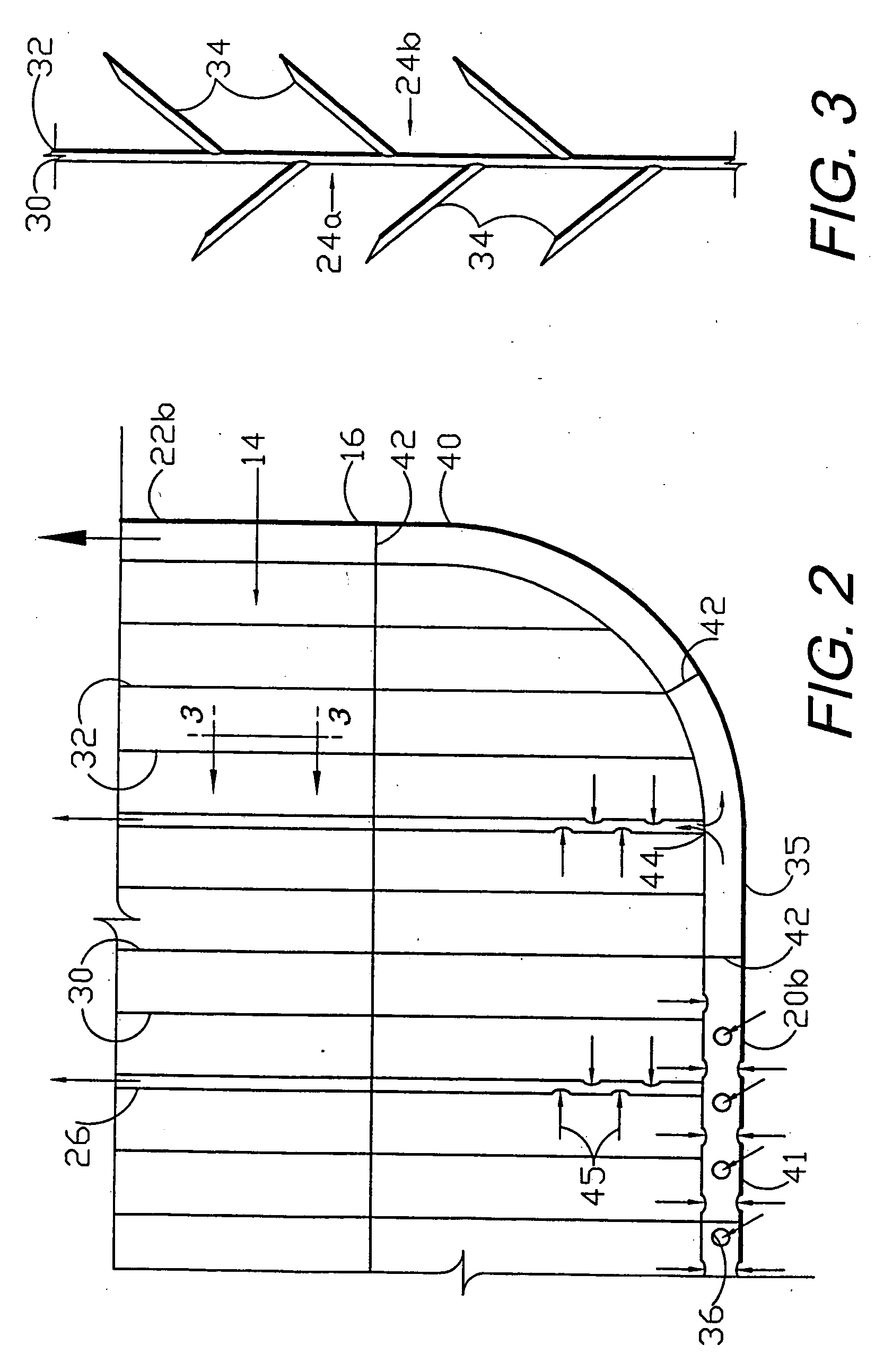
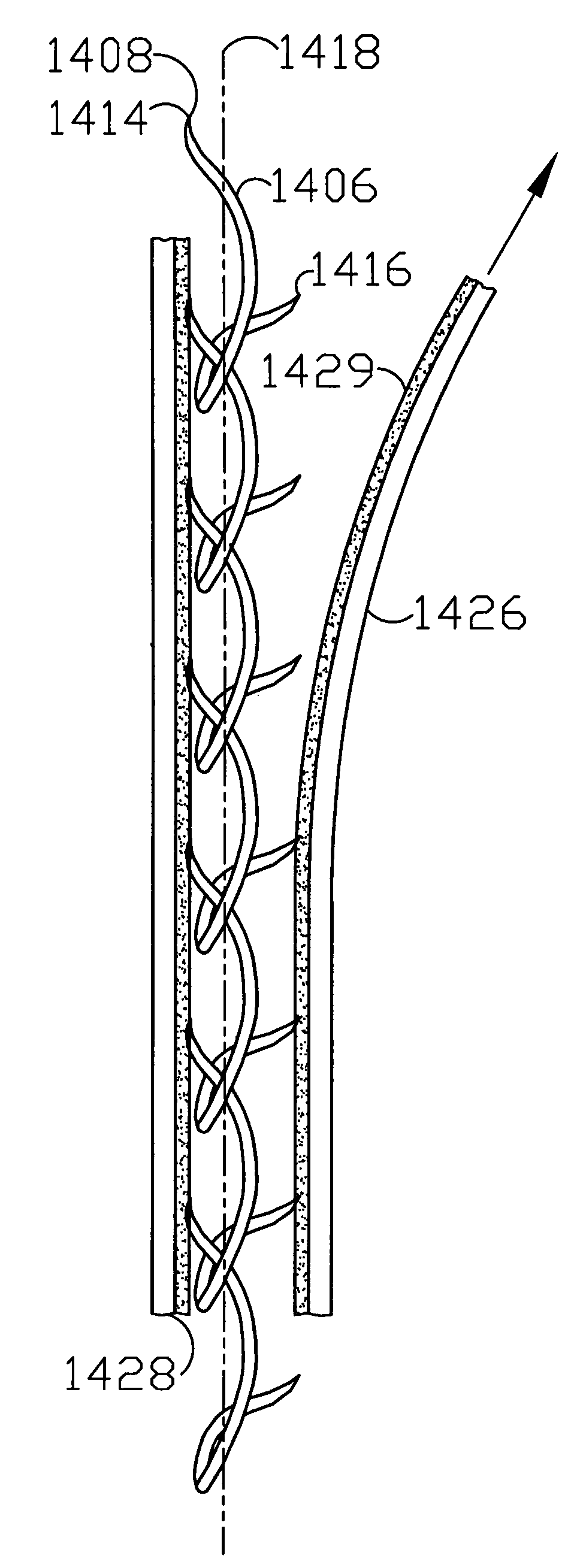
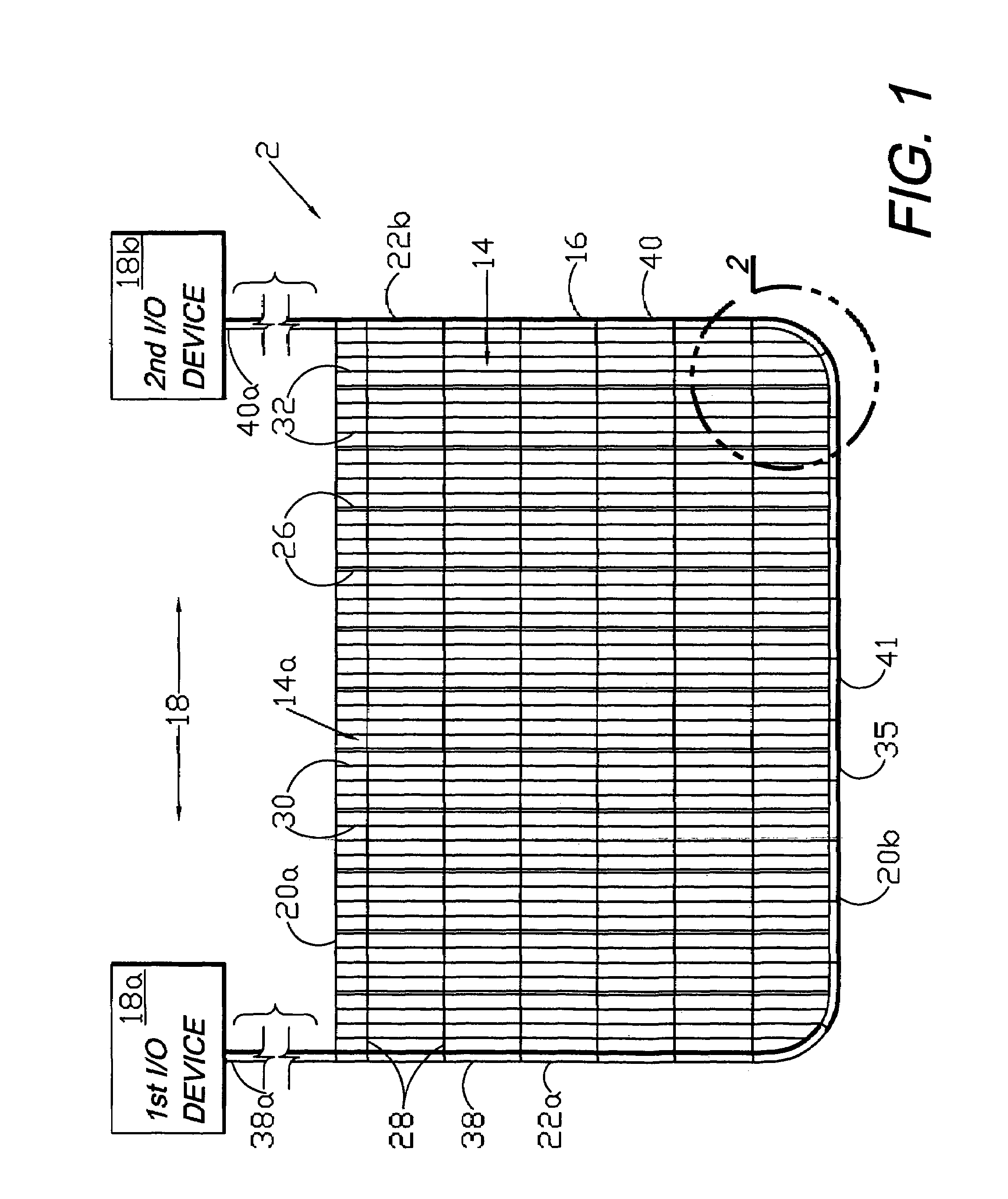
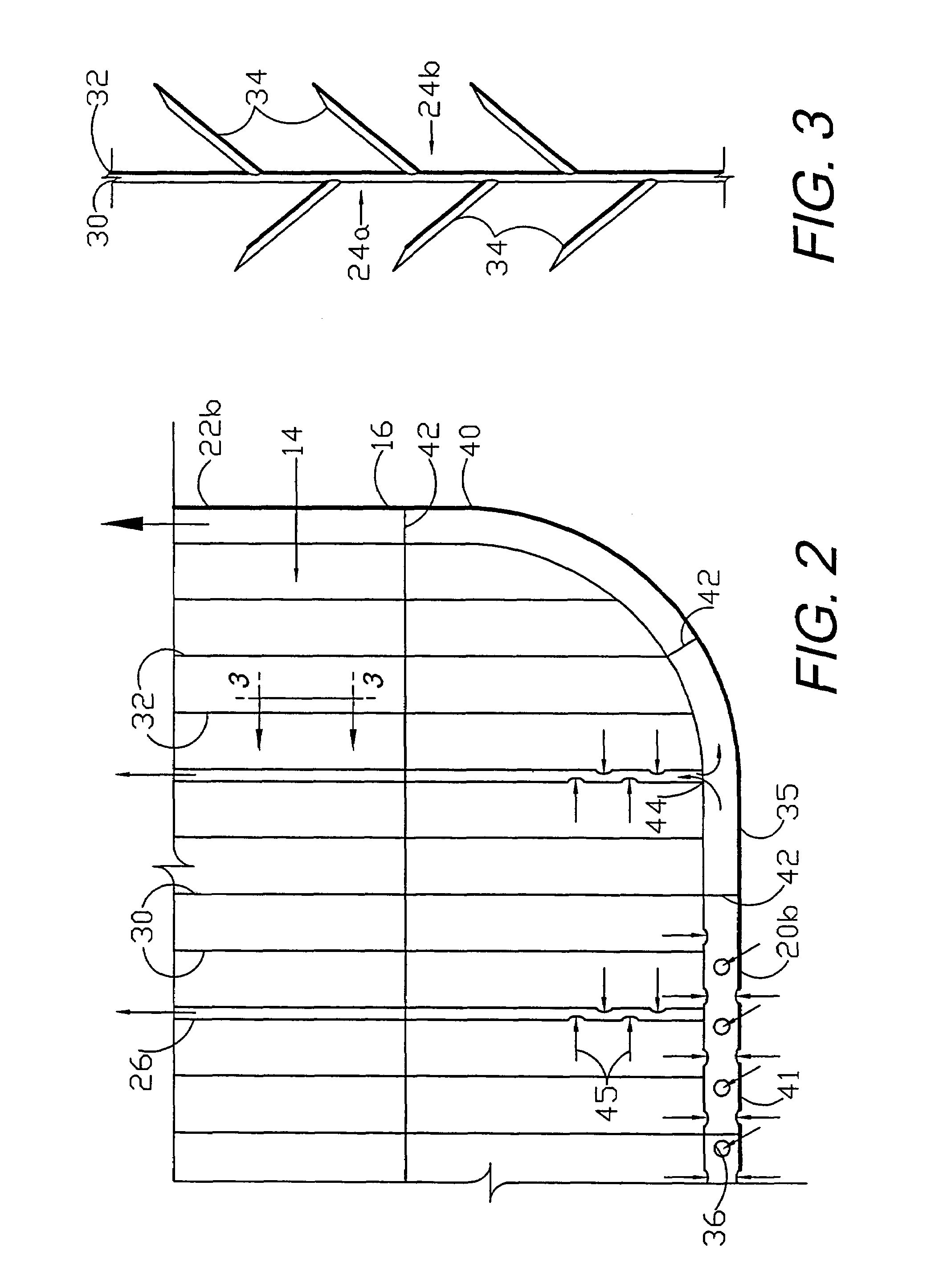
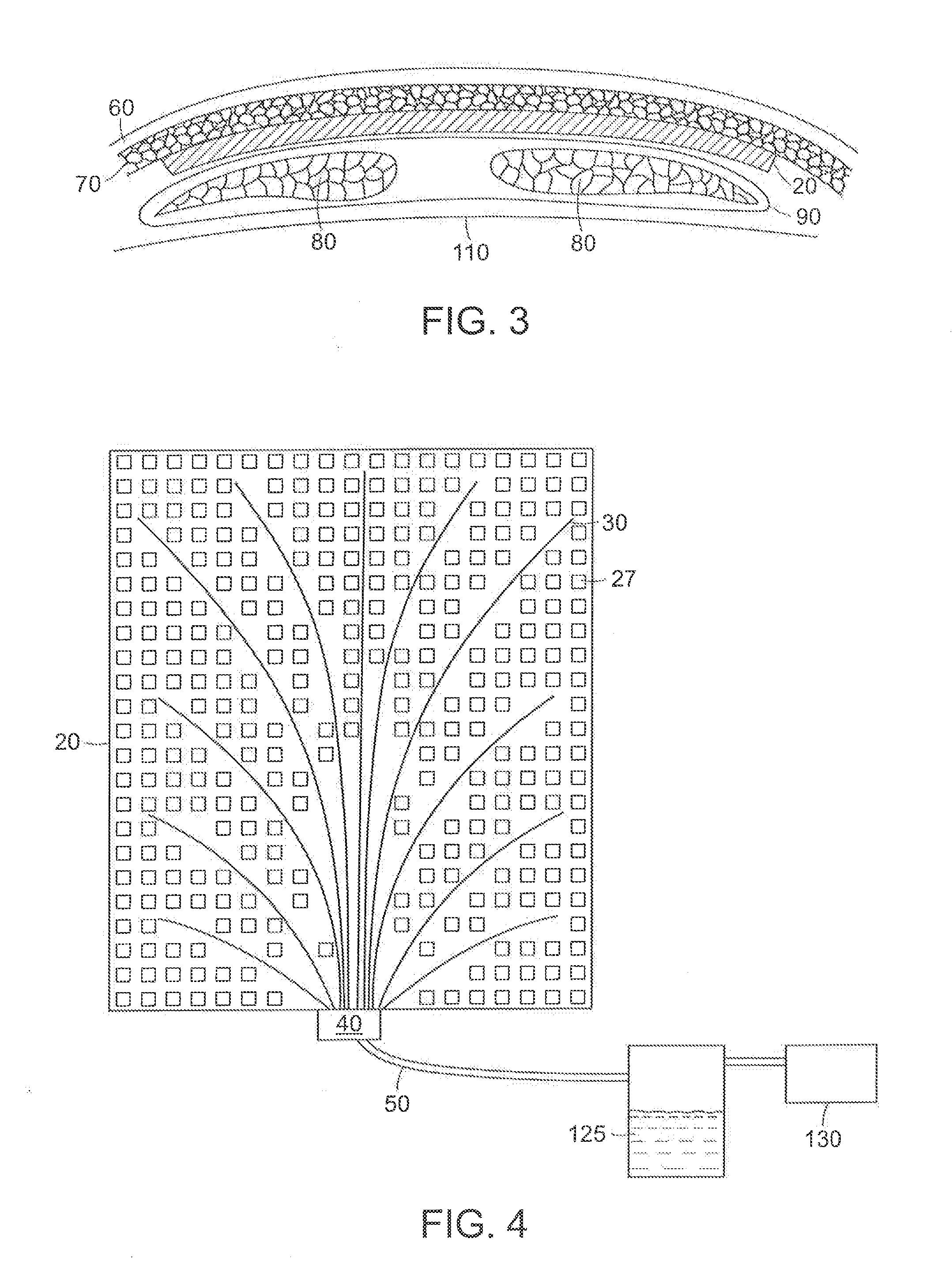
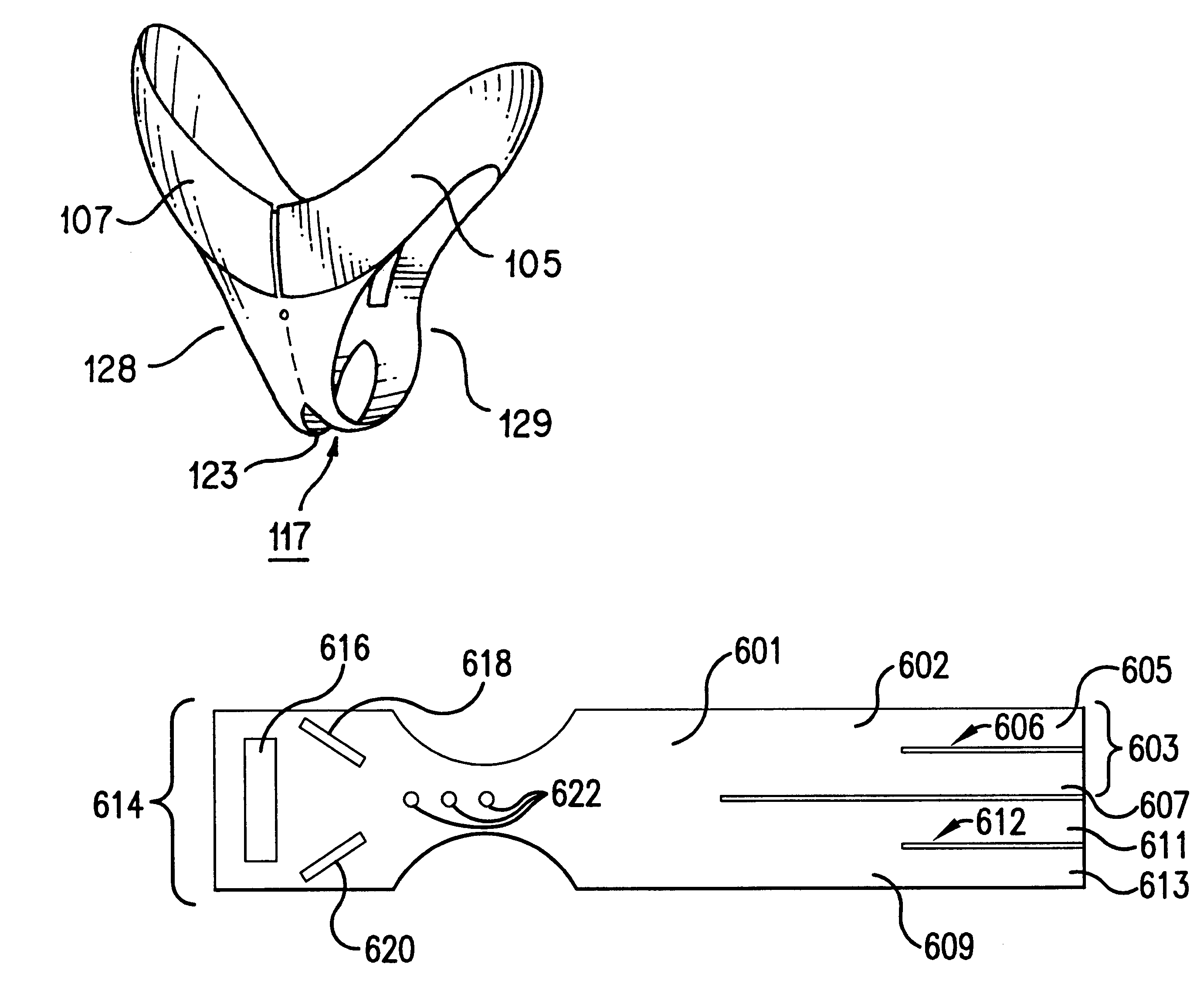
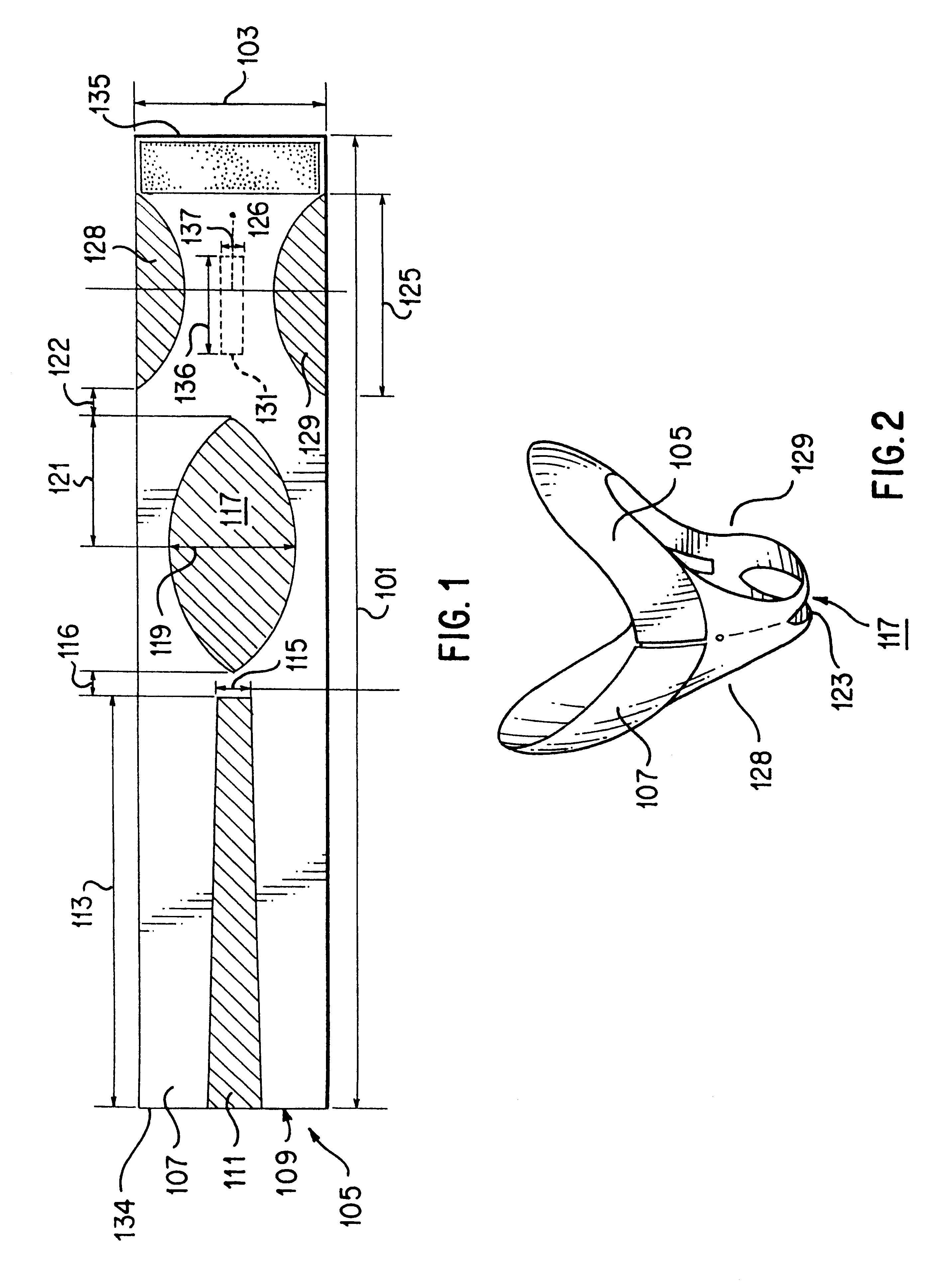
Circumferential medical closure device and method
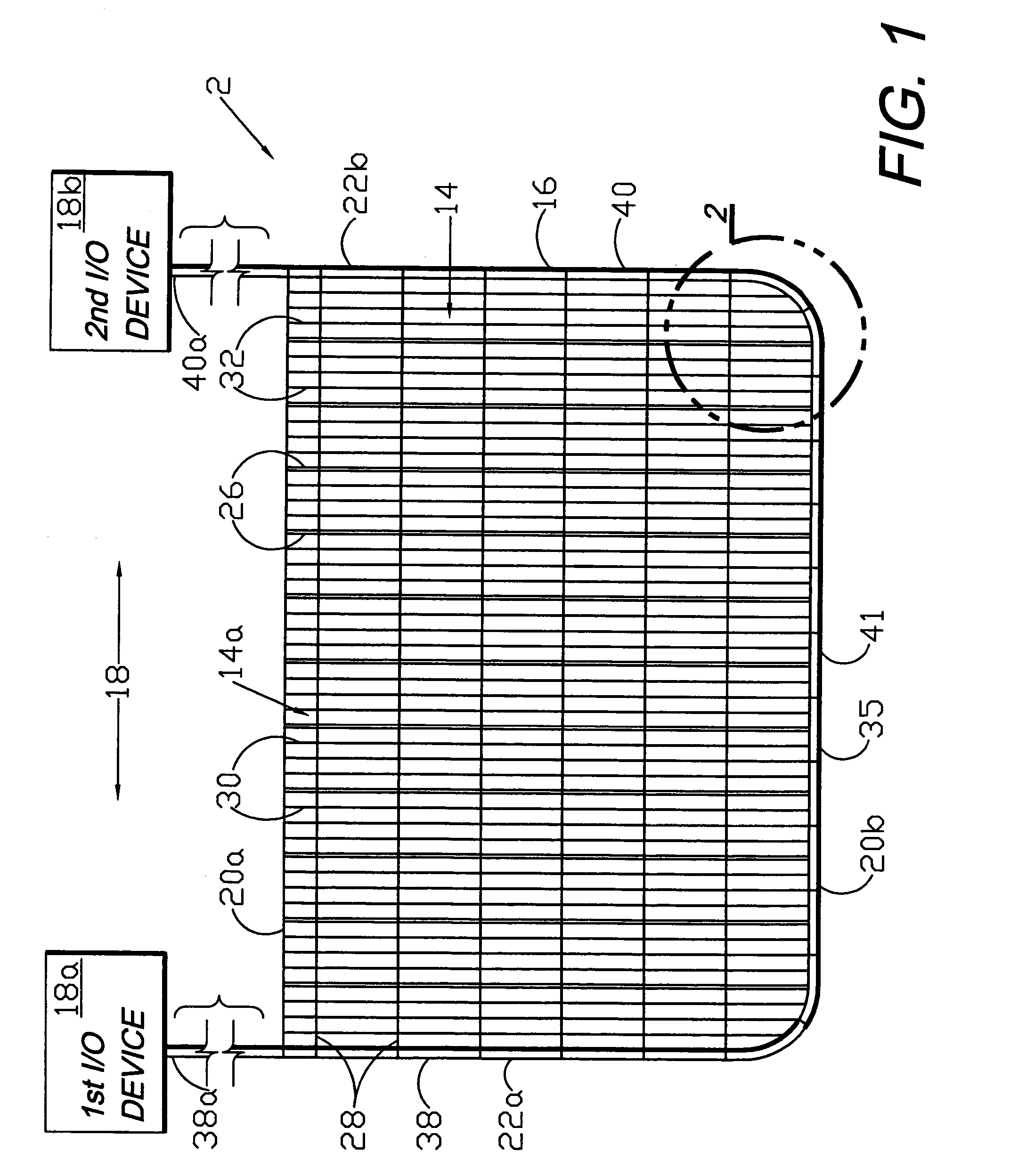
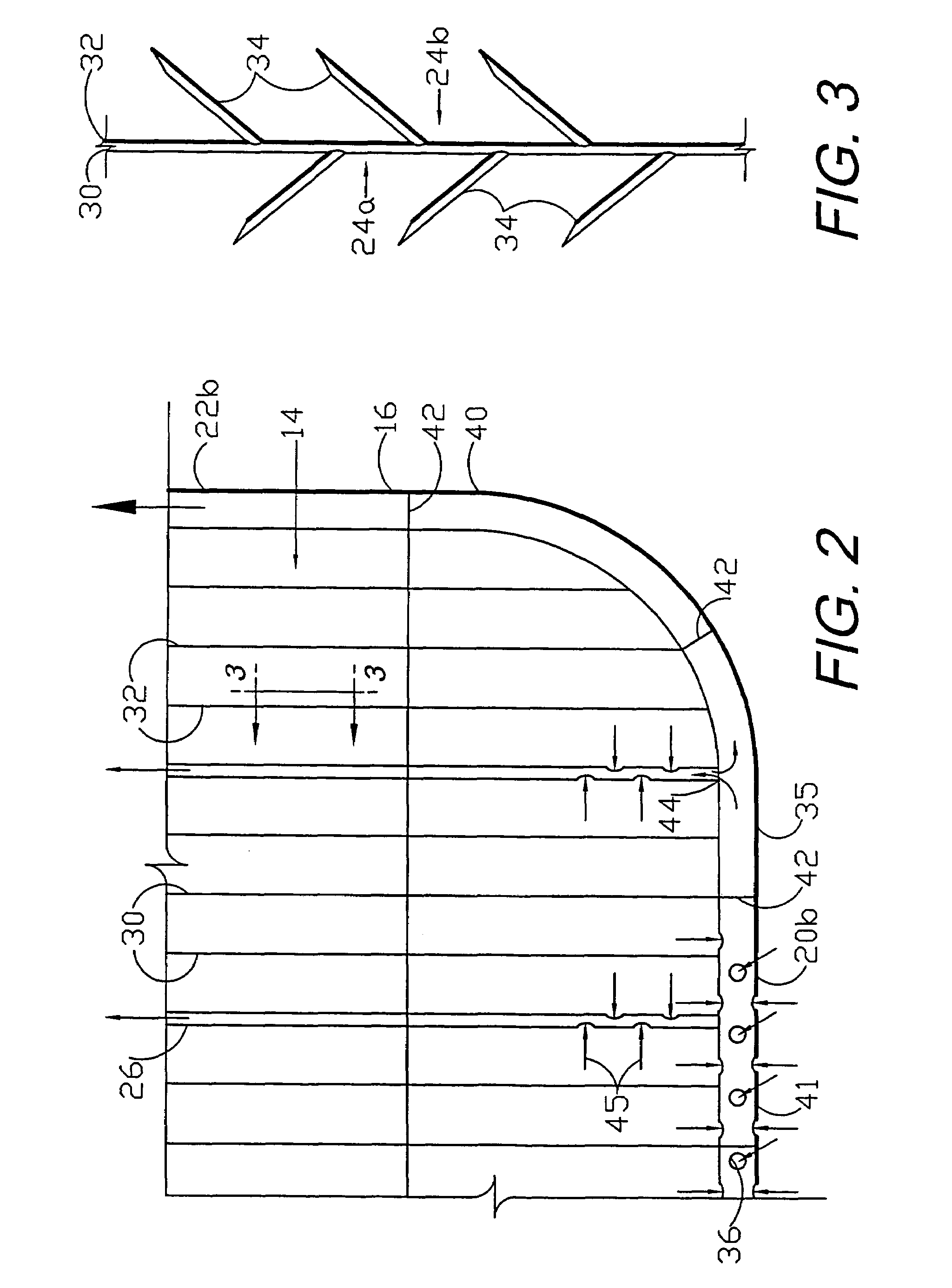
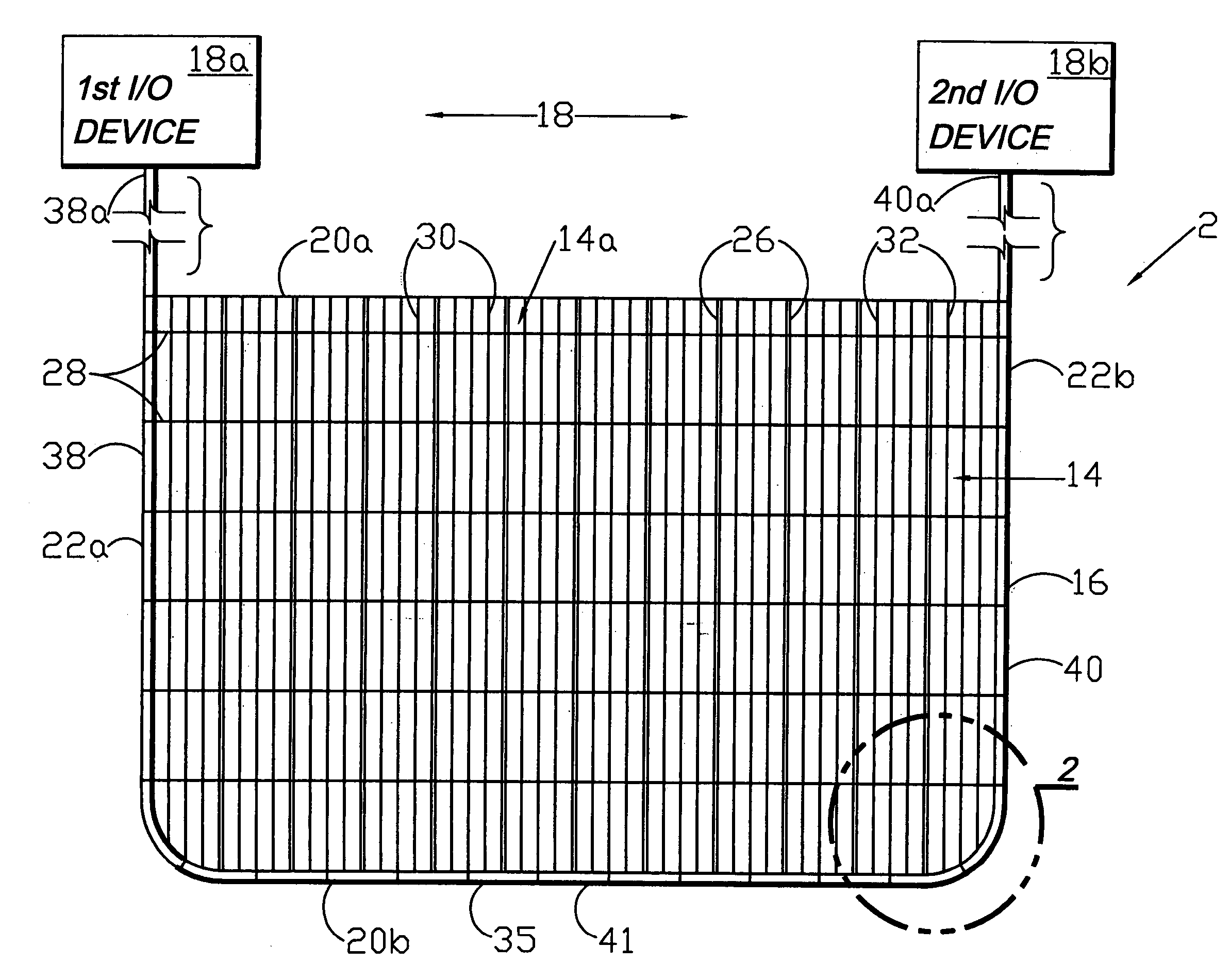
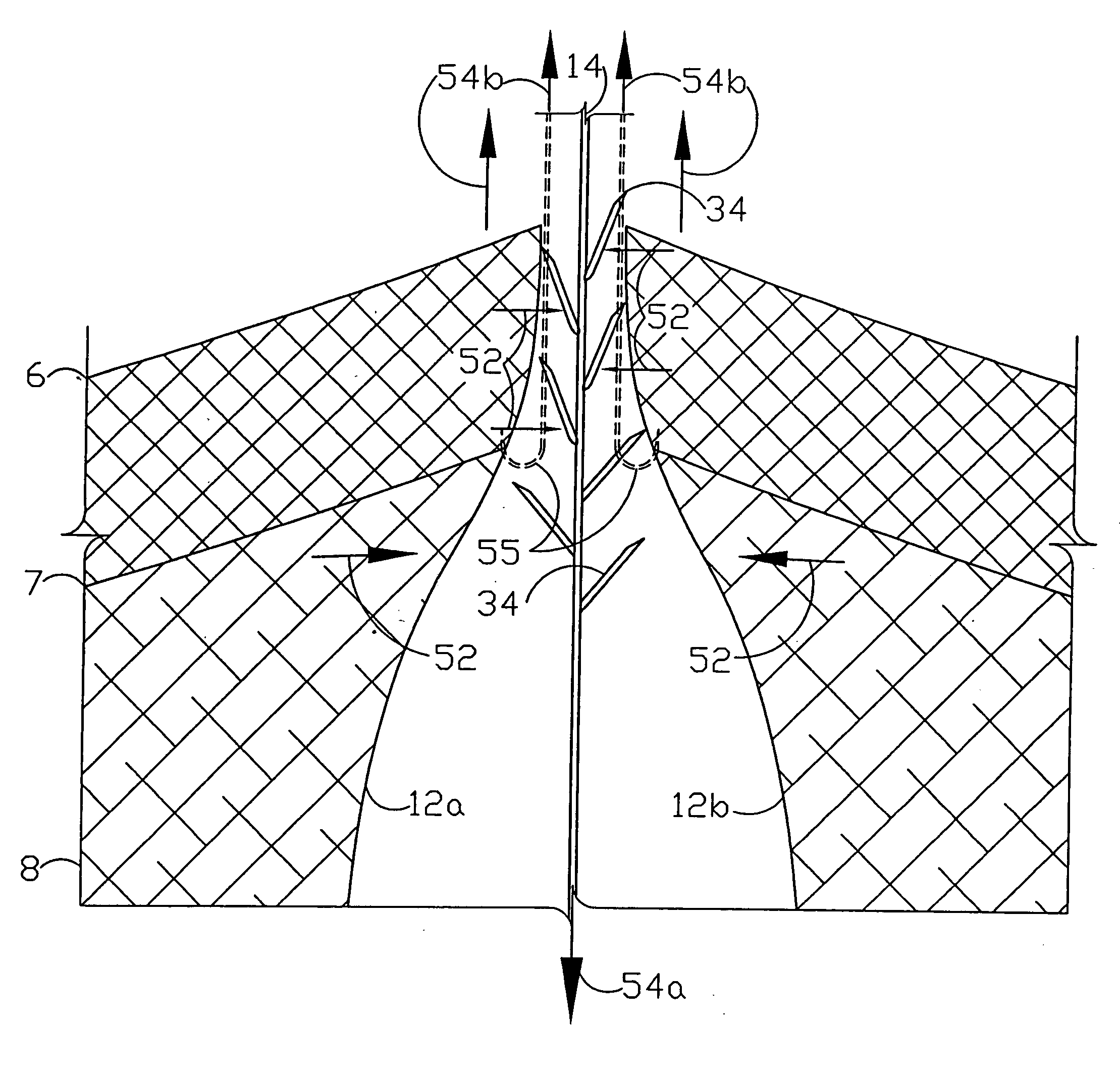
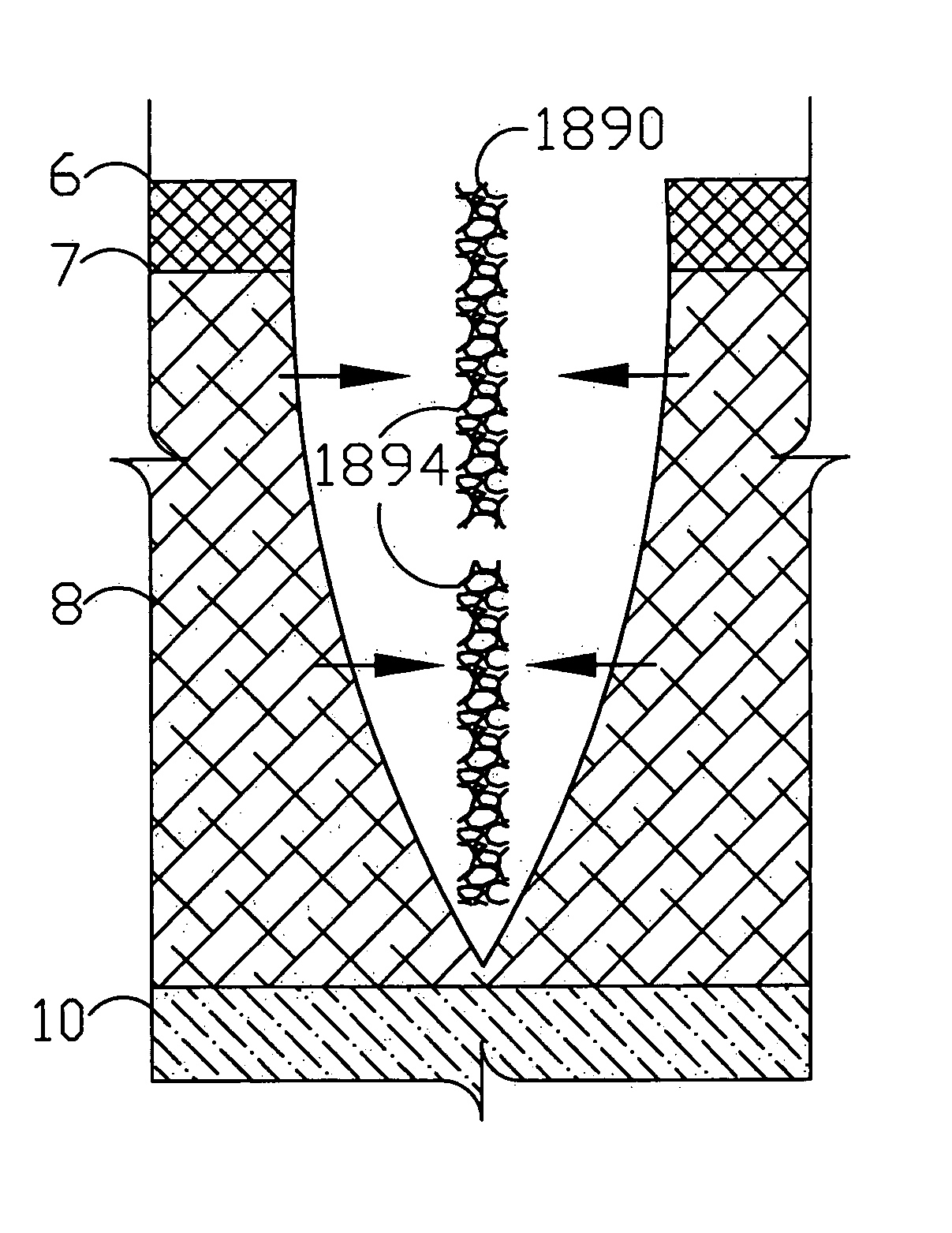
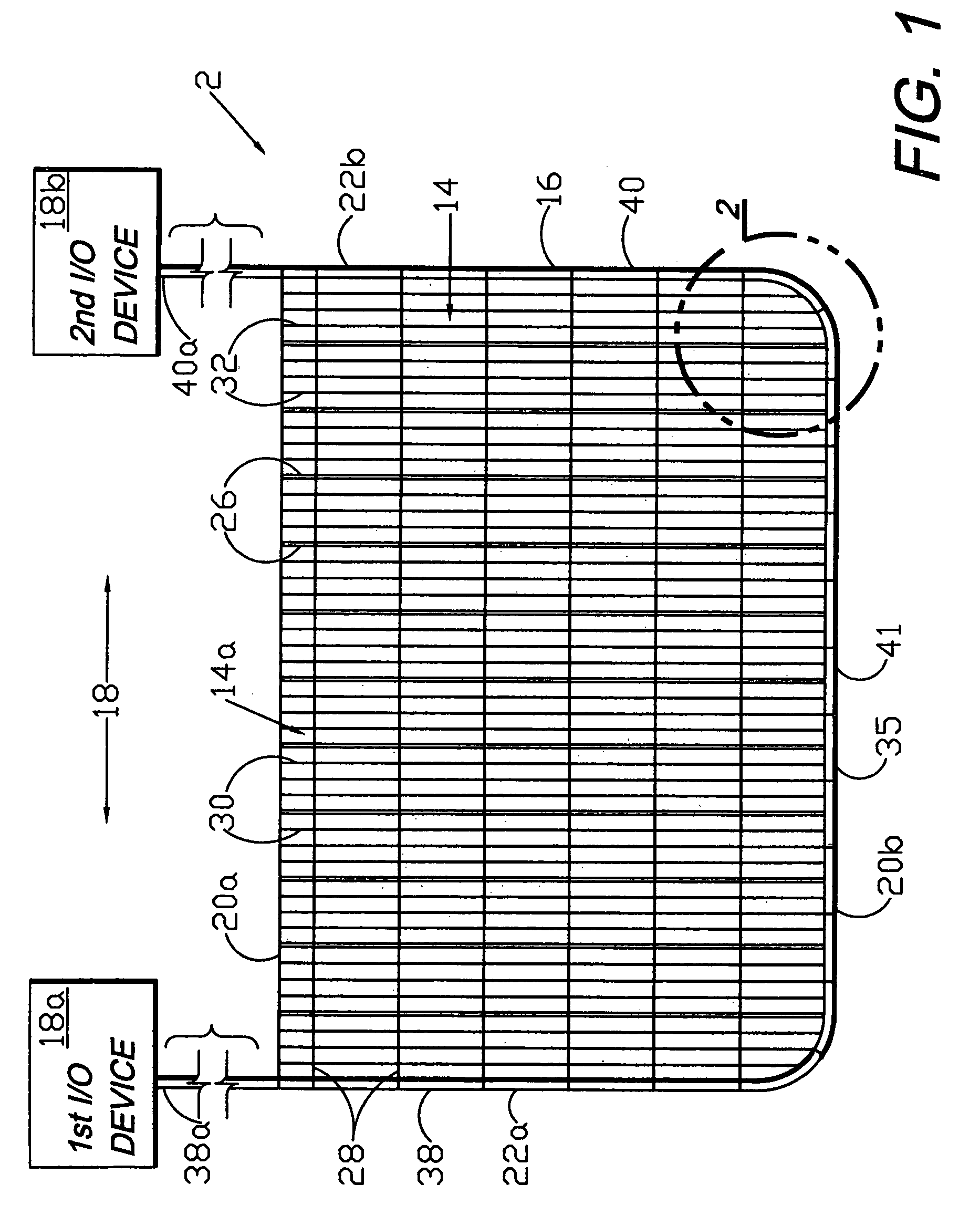
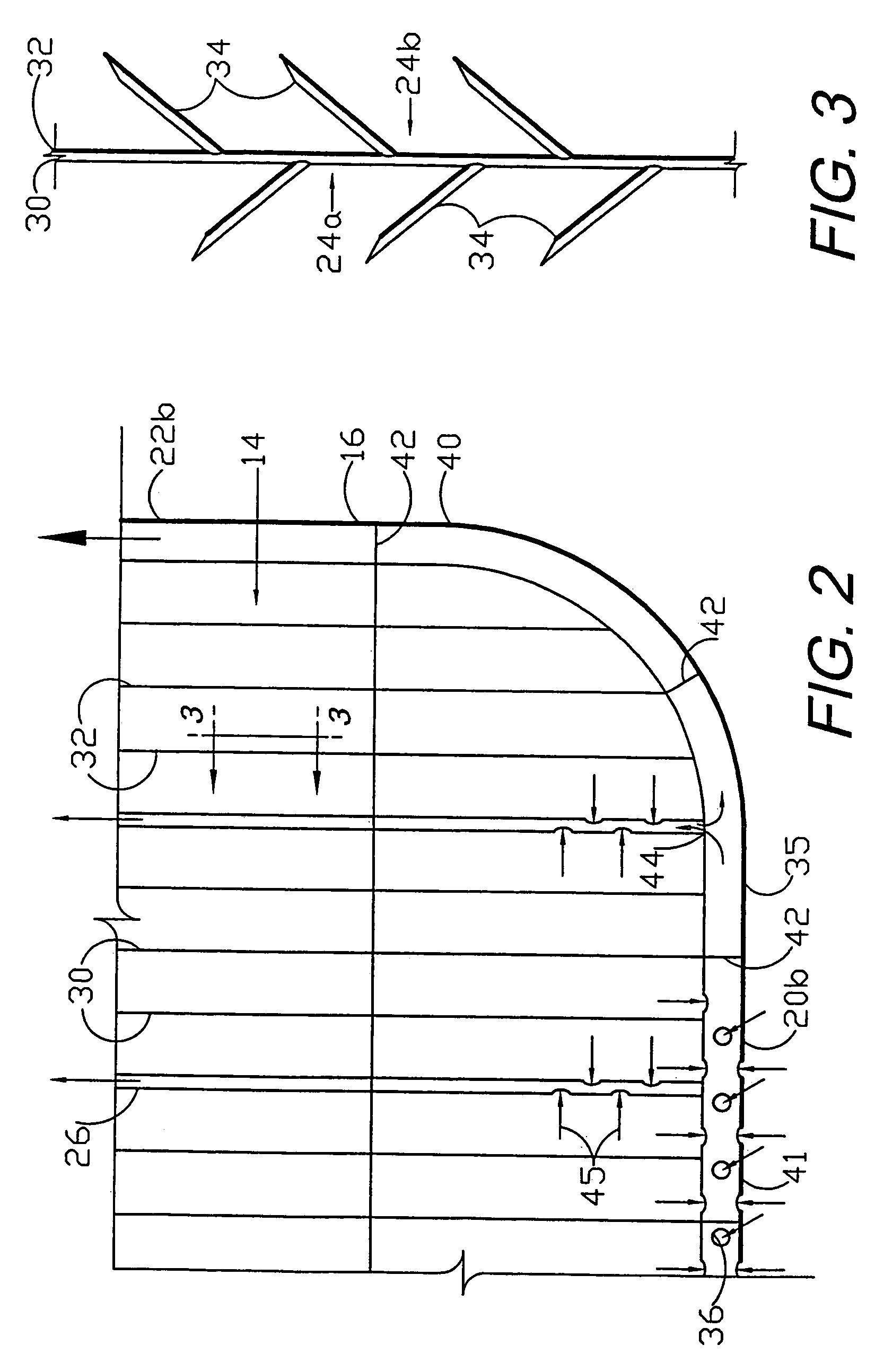
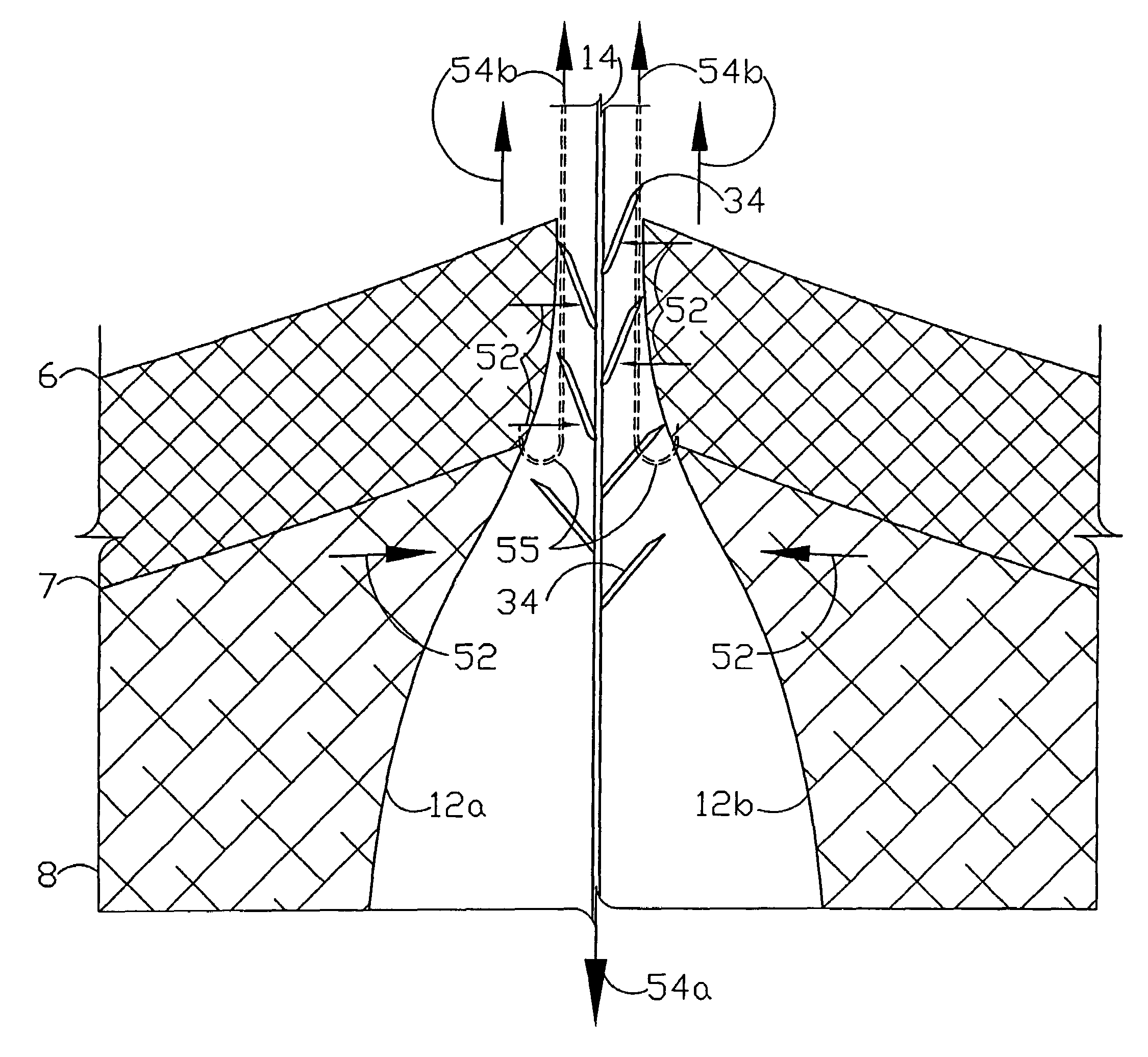
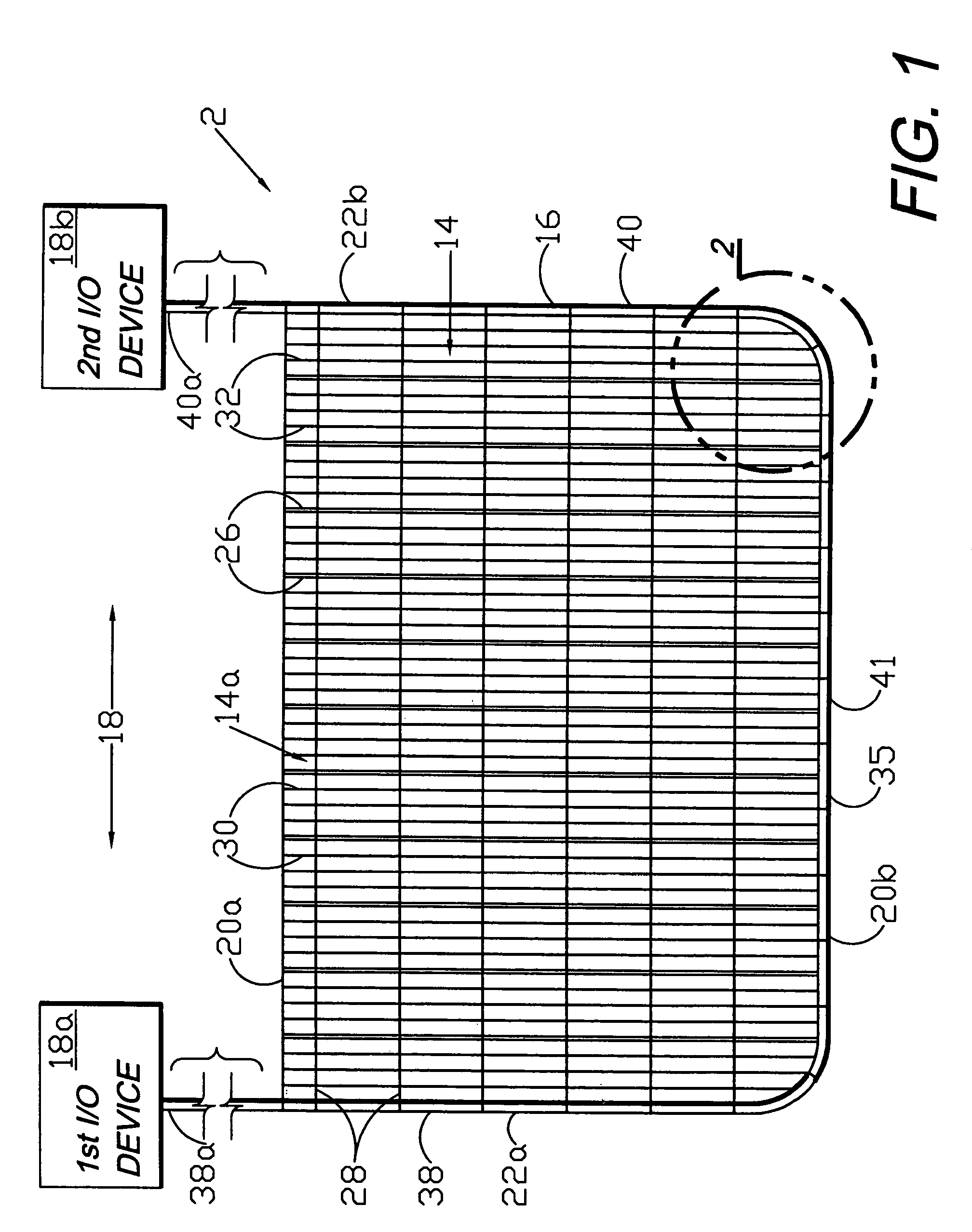
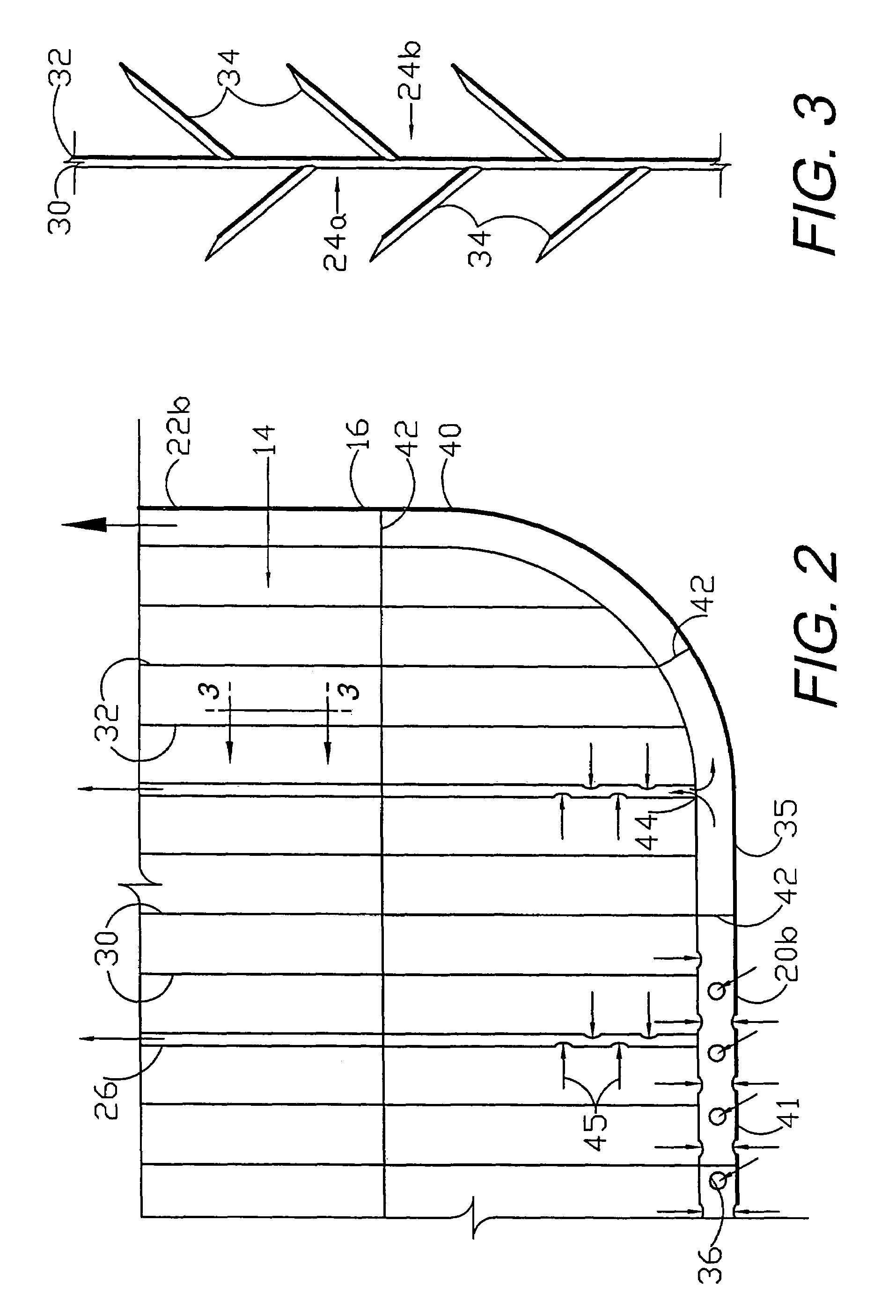
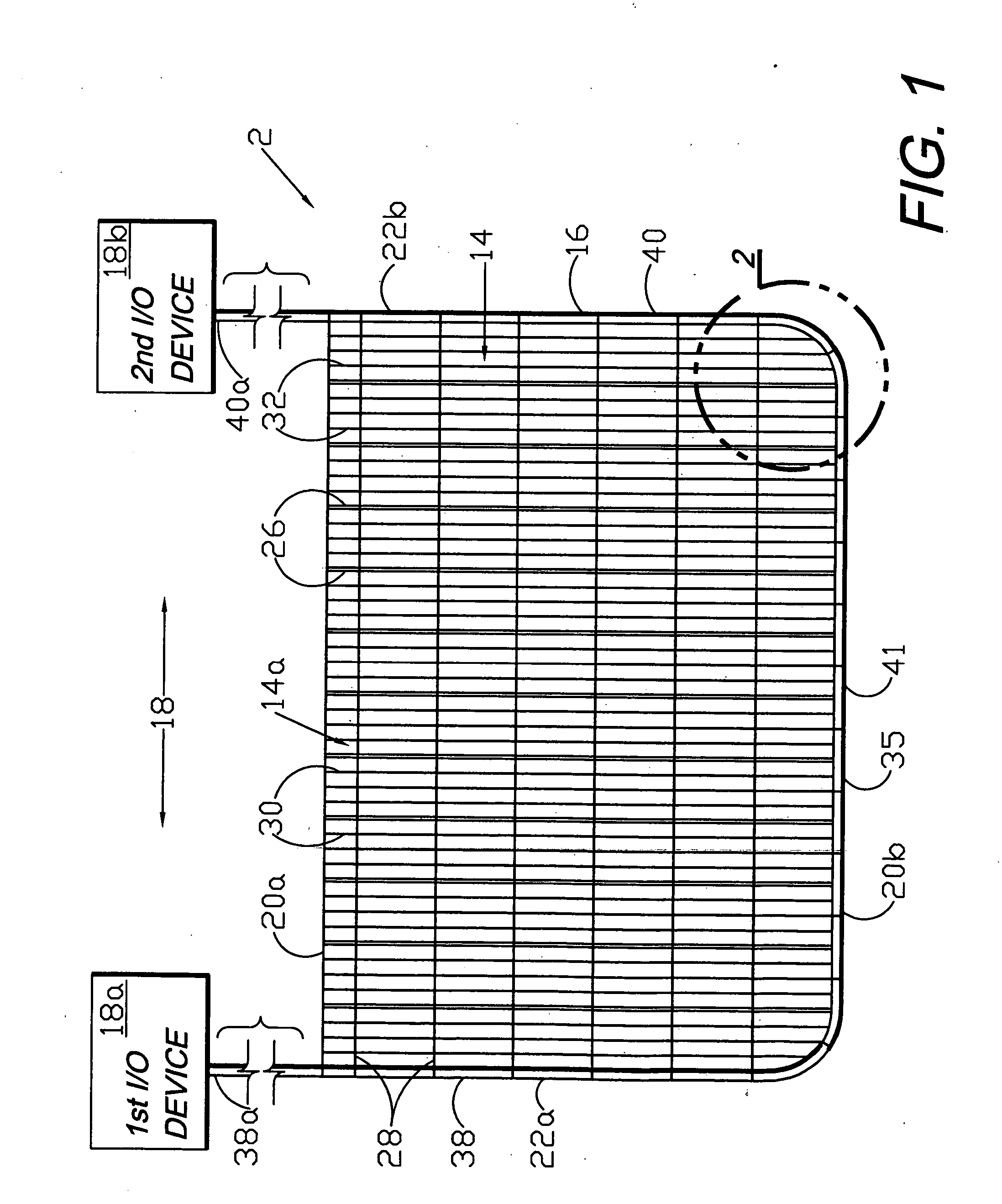
A flexible medical closure screen device for a separation of first and second tissue portions is provided, which includes a mesh screen comprising tubular vertical risers, vertical strands with barbed filaments, and horizontal spacers connecting the risers and strands in a grid-like configuration. An optional perimeter member partly surrounds the screen and can comprise a perimeter tube fluidically coupled with the vertical risers to form a tubing assembly. Various input / output devices can optionally be connected to the perimeter tube ends for irrigating and / or draining the separation according to methodologies of the present invention. Separation closure, irrigation and drainage methodologies are disclosed utilizing various combinations of closure screens, tubing, sutures, fluid transfer elements and gradient force sources. The use of mechanical forces associated with barbed strands for repositionably securing separated tissues together is disclosed. The use of same for eliminating or reducing the formation of subcutaneous voids or pockets, which can potentially form hematoma and seroma effects, is also disclosed. Alternative embodiments of the invention have circumferential configurations for approximating and closing separated tissue portions such as tendons, nerves and blood vessels. Tissue closure methods include the steps of circumferentially applying a screen to separated tissue portions and penetrating the tissue portions with prongs.
Owner:3M INNOVATIVE PROPERTIES CO
Circumferential medical closure device and method
A flexible medical closure screen device for a separation of first and second tissue portions is provided, which includes a mesh screen comprising tubular vertical risers, vertical strands with barbed filaments, and horizontal spacers connecting the risers and strands in a grid-like configuration. An optional perimeter member partly surrounds the screen and can comprise a perimeter tube fluidically coupled with the vertical risers to form a tubing assembly. Various input / output devices can optionally be connected to the perimeter tube ends for irrigating and / or draining the separation according to methodologies of the present invention. Separation closure, irrigation and drainage methodologies are disclosed utilizing various combinations of closure screens, tubing, sutures, fluid transfer elements and gradient force sources. The use of mechanical forces associated with barbed strands for repositionably securing separated tissues together is disclosed. The use of same for eliminating or reducing the formation of subcutaneous voids or pockets, which can potentially form hematoma and seroma effects, is also disclosed. Alternative embodiments of the invention have circumferential configurations for approximating and closing separated tissue portions such as tendons, nerves and blood vessels. Tissue closure methods include the steps of circumferentially applying a screen to separated tissue portions and penetrating the tissue portions with prongs.
Owner:3M INNOVATIVE PROPERTIES CO
Medical closure screen installation systems and methods
InactiveUS20050177190A1Easy to drainReduce and eliminate formationSuture equipmentsStaplesVertical tubeWater irrigation
A medical closure screen device for a separation of first and second tissue portions is provided, which includes a mesh screen comprising tubular vertical risers, vertical strands with barbed filaments, and horizontal spacers connecting the risers and strands in a grid-like configuration. An optional perimeter member partly surrounds the screen and can comprise a perimeter tube fluidically coupled with the vertical risers to form a tubing assembly. Various input / output devices can optionally be connected to the perimeter tube ends for irrigating and / or draining the separation according to methodologies of the present invention. Separation closure, irrigation and drainage methodologies are disclosed utilizing various combinations of closure screens, tubing, sutures, fluid transfer elements and gradient force sources. The use of mechanical forces associated with barbed strands for repositionably securing separated tissues together is disclosed. The use of same for eliminating or reducing the formation of subcutaneous voids or pockets, which can potentially form hematoma and seroma effects, is also disclosed. Further disclosed are alternative embodiment medical closure screen installation systems and methods.
Owner:3M INNOVATIVE PROPERTIES CO
Internal and external medical closure screen systems and methods
InactiveUS8062331B2Reduce and eliminate formationEasy to drainSuture equipmentsDiagnosticsVertical tubeSeroma
An internal and external medical closure system for a separation of first and second tissue portions is provided, which includes a mesh screen comprising tubular vertical risers, vertical strands with barbed filaments, and horizontal spacers connecting the risers and strands in a grid-like configuration. An optional perimeter member partly surrounds the screen and can comprise a perimeter tube fluidically coupled with the vertical risers to form a tubing assembly. Various input / output devices can optionally be connected to the perimeter tube ends for irrigating and / or draining the separation according to methodologies of the present invention. Separation closure, irrigation and drainage methodologies are disclosed utilizing various combinations of closure screens, tubing, sutures, fluid transfer elements and gradient force sources. The use of mechanical forces associated with barbed strands for repositionably securing separated tissues together is disclosed. The use of same for eliminating or reducing the formation of subcutaneous voids or pockets, which can potentially form hematoma and seroma effects, is also disclosed. Further disclosed are alternative embodiment medical closure screen installation systems and methods.
Owner:3M INNOVATIVE PROPERTIES CO
Flexible medical closure screen and method
Owner:3M INNOVATIVE PROPERTIES CO
Medical closure screen installation systems and methods
InactiveUS7413570B2Reduce and eliminate formationEasy to drainSuture equipmentsStaplesVertical tubeSeroma
Owner:3M INNOVATIVE PROPERTIES CO
Medical closure clip system and method
A medical closure screen device for a separation of first and second tissue portions is provided, which includes a mesh screen comprising tubular vertical risers, vertical strands with barbed filaments, and horizontal spacers connecting the risers and strands in a grid-like configuration. An optional perimeter member partly surrounds the screen and can comprise a perimeter tube fluidically coupled with the vertical risers to form a tubing assembly. Various input / output devices can optionally be connected to the perimeter tube ends for irrigating and / or draining the separation according to methodologies of the present invention. Separation closure, irrigation and drainage methodologies are disclosed utilizing various combinations of closure screens, tubing, sutures, fluid transfer elements and gradient force sources. The use of mechanical forces associated with barbed strands for repositionably securing separated tissues together is disclosed. The use of same for eliminating or reducing the formation of subcutaneous voids or pockets, which can potentially form hematoma and seroma effects, is also disclosed. The device can be fabricated and the method practiced with clips having various configurations.
Owner:3M INNOVATIVE PROPERTIES CO
Flexible medical closure screen and method
InactiveUS20050240220A1Reduce and eliminate formationSuture equipmentsStaplesVertical tubeEngineering
A flexible medical closure screen for closing a separation of first and second tissue portions is provided, which includes a mesh screen comprising tubular vertical risers, vertical strands with barbed filaments, and horizontal spacers connecting the risers and strands in a grid-like configuration. An optional perimeter member partly surrounds the screen and can comprise a perimeter tube fluidically coupled with the vertical risers to form a tubing assembly. Various input / output devices can optionally be connected to the perimeter tube ends for irrigating and / or draining the separation according to methodologies of the present invention. Separation closure, irrigation and drainage methodologies are disclosed utilizing various combinations of closure screens, tubing, sutures, fluid transfer elements and gradient force sources. The use of mechanical forces associated with barbed strands for repositionably securing separated tissues together is disclosed. The use of same for eliminating or reducing the formation of subcutaneous voids or pockets, which can potentially form hematoma and seroma effects, is also disclosed. Alternative embodiment flexible closure screens and methods of using same are also disclosed.
Owner:KCI LICENSING INC
Medical closure clip system and method
Owner:3M INNOVATIVE PROPERTIES CO
Tissue treatments with adipocyte cells
InactiveUS7767452B2Reduce the possibilityLower potentialBiocidePeptide/protein ingredientsWrinkle skinNasolabial fold
Certain embodiments here in are directed to a method of treating a tissue associated with a defect in a human including wrinkles, rhytids, depressed scar, cutaneous depressions, stretch marks, hyperplasia of the lip, nasolabial fold, melolabial fold, scarring from acne vulgaris, and post-rhinoplasty irregularity. The tissue defect may be treated by introducing a plurality of in vitro cultured autologous fibroblast cells at or proximal to the defect area of the patient's tissue. The autologous fibroblast cells may have been cultured in vitro to expand the number of fibroblast cells in at least one medium that comprises autologous serum. The autologous fibroblast cell cultures may be derived from connective tissue, dermal, fascial fibroblasts, papillary fibroblasts, and / or reticular fibroblasts.
Owner:KLEINSEK DON A
Method for chondrocyte expansion with phenotype retention
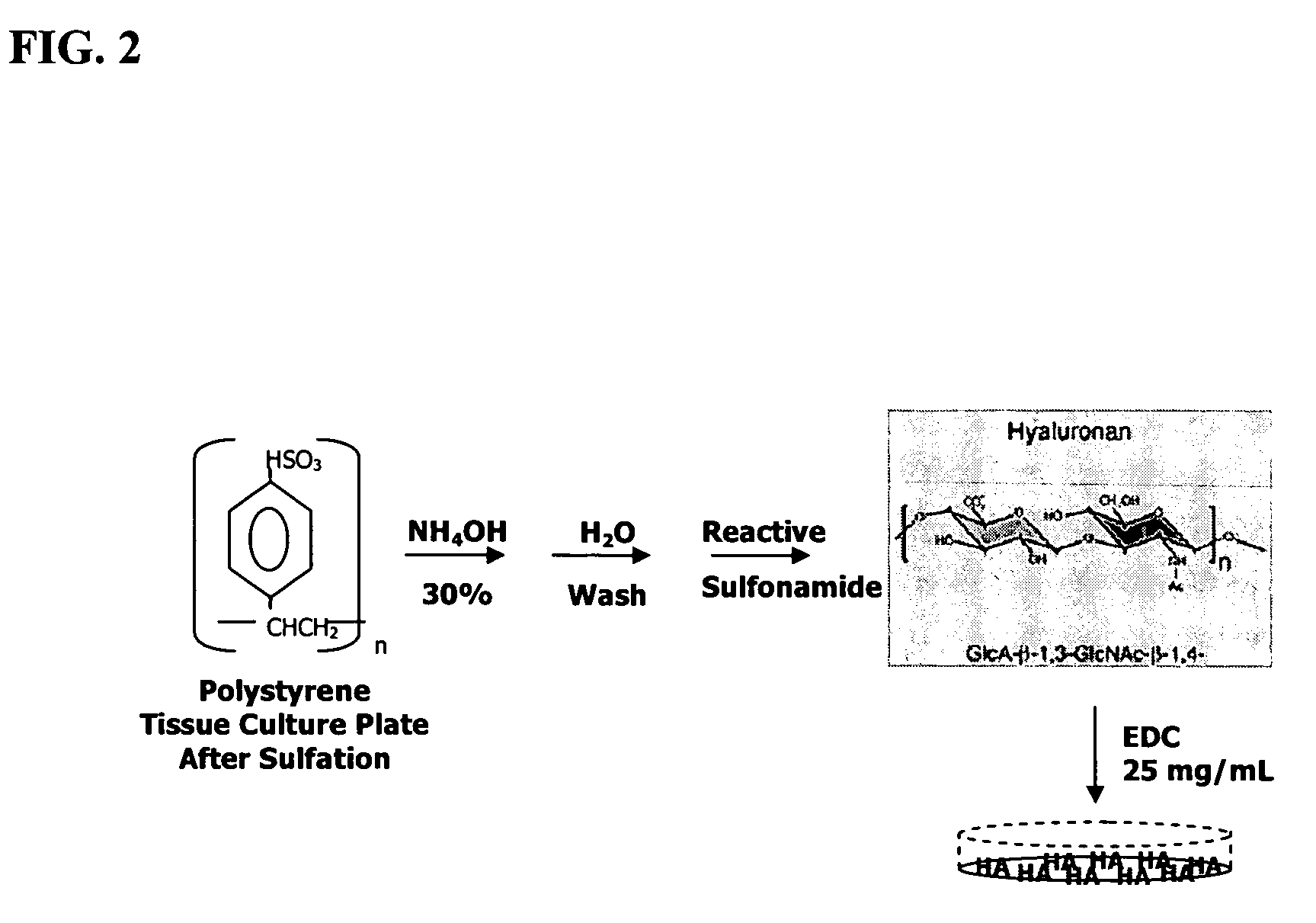
The present invention provides a method that maintains chondrocyte phenotype during serial expansion by culturing a population of chondrocytes in a defined serum-free culture medium containing cytokines and on a substrate that is modified by covalent attachment of hyaluronic acid. The underlying principle is to maintain native chondrocyte phenotype by growing the dissociated chondrocytes on a substrate modified by covalent attachment of hyaluronic acid to retain native chondrocyte morphology and function. Chondrocyte expanded in this manner can be used in various medical applications to repair cartilaginous tissues that have been injured by trauma or disease. This substratum provides a microenvironment that more closely mimics that of native articular cartilage, thereby promoting chondrogenesis in a predictable manner.
Owner:ZIMMER INC +1
Surgical cavity drainage and closure system
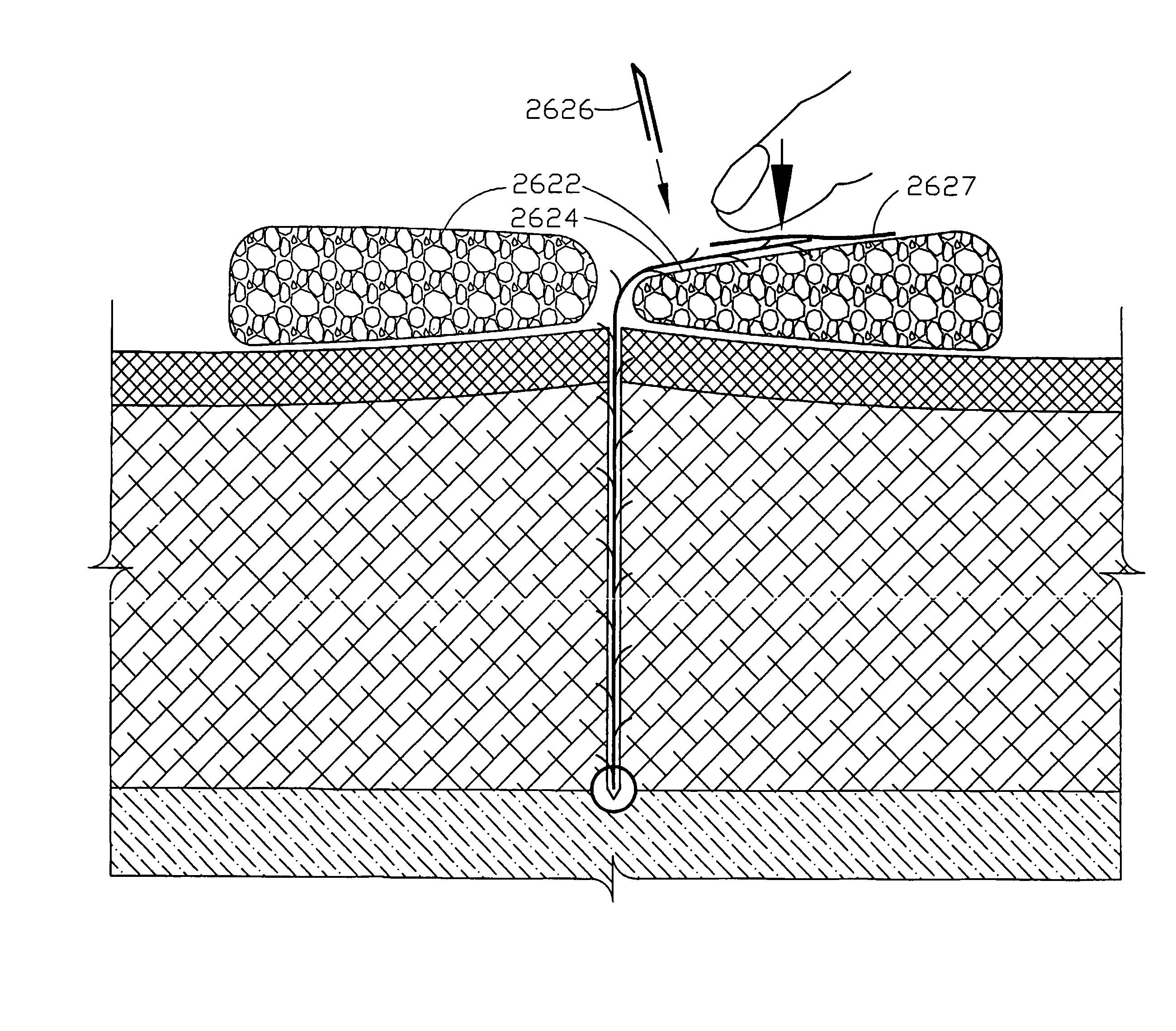
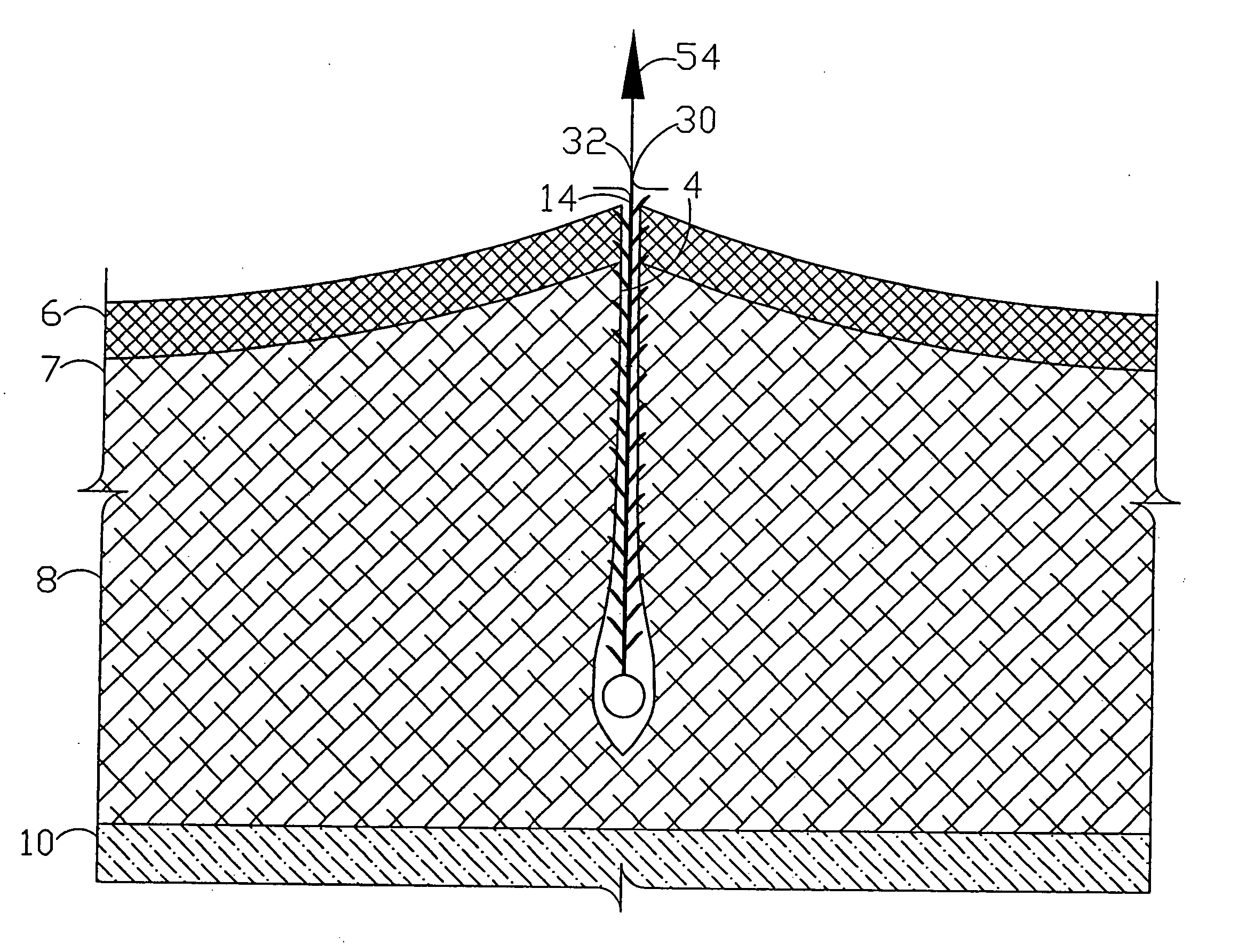
ActiveUS20130274717A1Treating and preventing seromaGood drainageUltrasonic/sonic/infrasonic diagnosticsSuture equipmentsWound dressingSurgical department
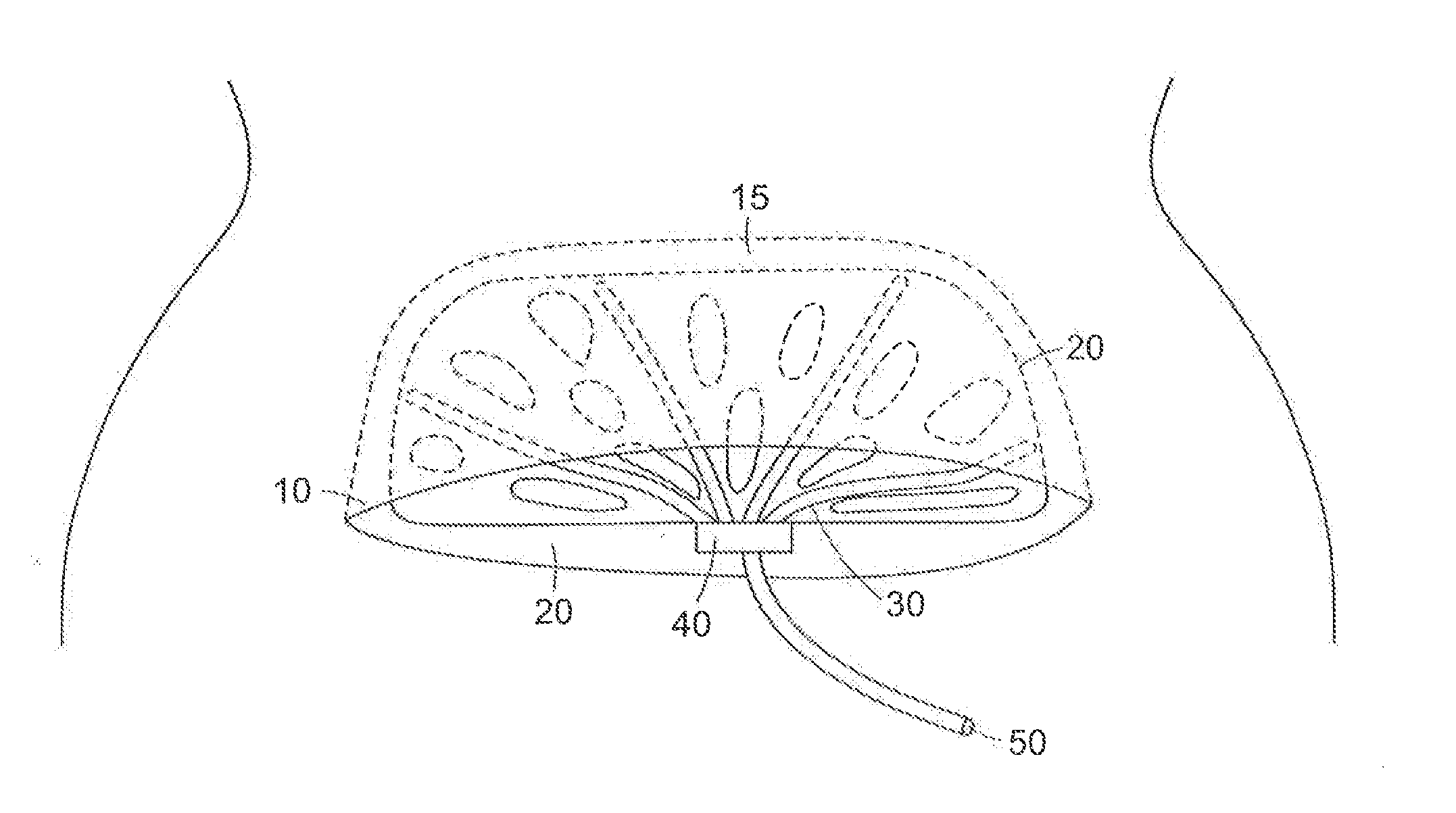
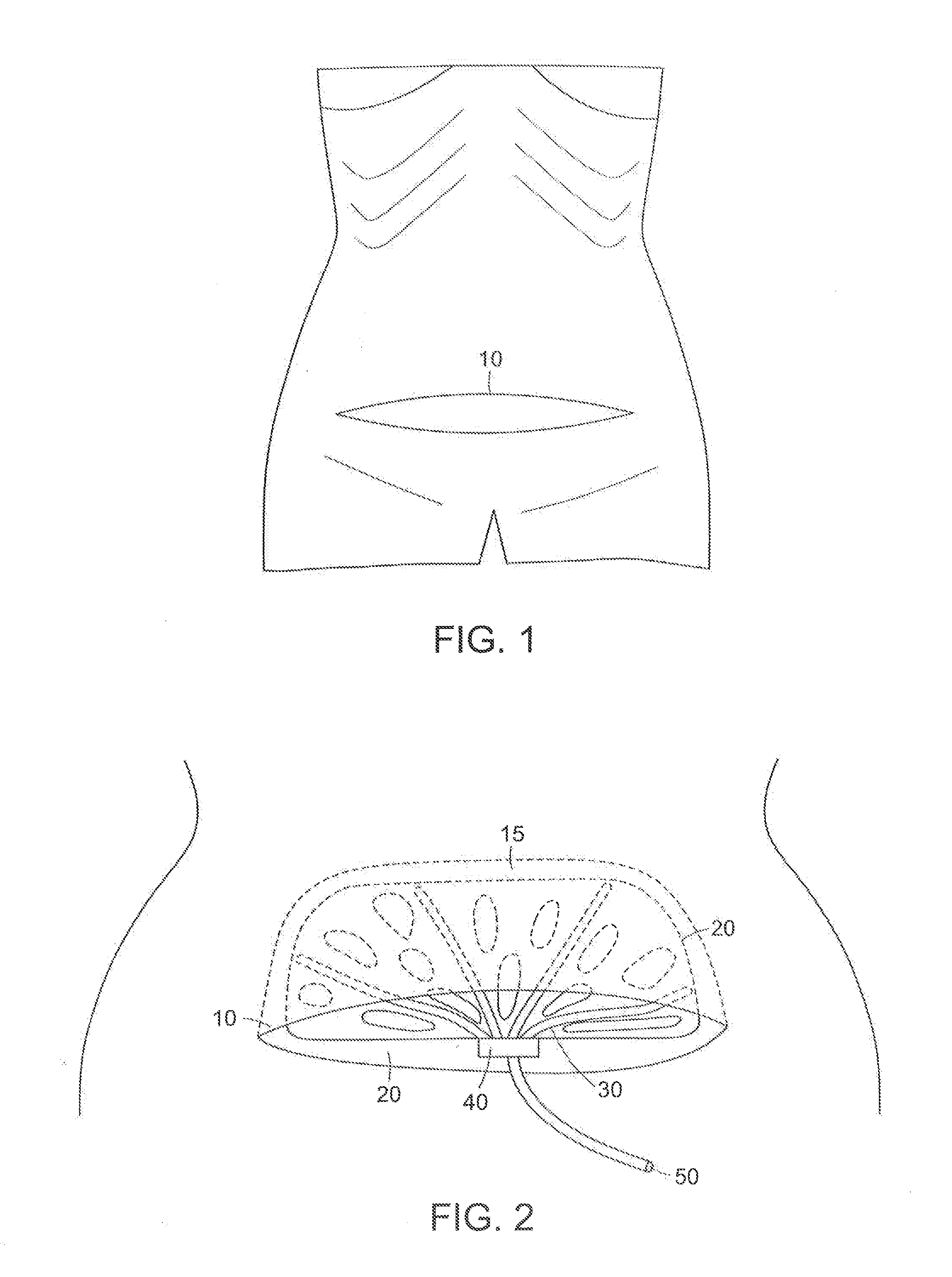
A surgical drain device includes a matrix of biodegradable polymer material and a plurality of drain tubes attached to the matrix. The device is implanted within a surgical wound to treat the presence of seromas, for example, and is used to promote drainage, tissue adhesion, and wound closure. The drain tubes converge into a common collection tube that leads wound fluid outside the body under gravity feed or negative pressure applied to the collection tube. The matrix contains an array of apertures that allow tissue contact across the device. A preferred embodiment comprises a tissue anchoring system including anchor elements such as hooks or barbs. The device can be used with a negative pressure system to further improve the drainage band can also be used with a wound dressing. The device and systems containing the device are particularly useful to promote the healing of surgical wounds from abdominal surgery.
Owner:UNIV OF MASSACHUSETTS
Compositions and methods for modular soft tissue repair
InactiveUS20100098739A1Superior in vivo engraftmentSuperior in tissue integrationBiocideMammal material medical ingredientsSerum free mediaTissue repair
Adipose tissue-derived stromal cells induced to form multicellular aggregates can be formed reliably and consistently, they can be maintained for prolonged periods in adherent or suspension culture, and they are able to survive, grow, and / or differentiate in serum-free media conditions. The present invention provides compositions and methods for the use of such aggregates in tissue repair. This culture platform provides a controlled and defined system in which to study and standardize adult stem cell biology, and begets an instinctive “modular” approach to the predictable replacement and regeneration of adipose tissue.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Methods for producing retinal tissue and retina-related cell
ActiveUS20140341864A1Improve efficiencySolve low usageBiocideNervous system cellsSerum free mediaNeural cell
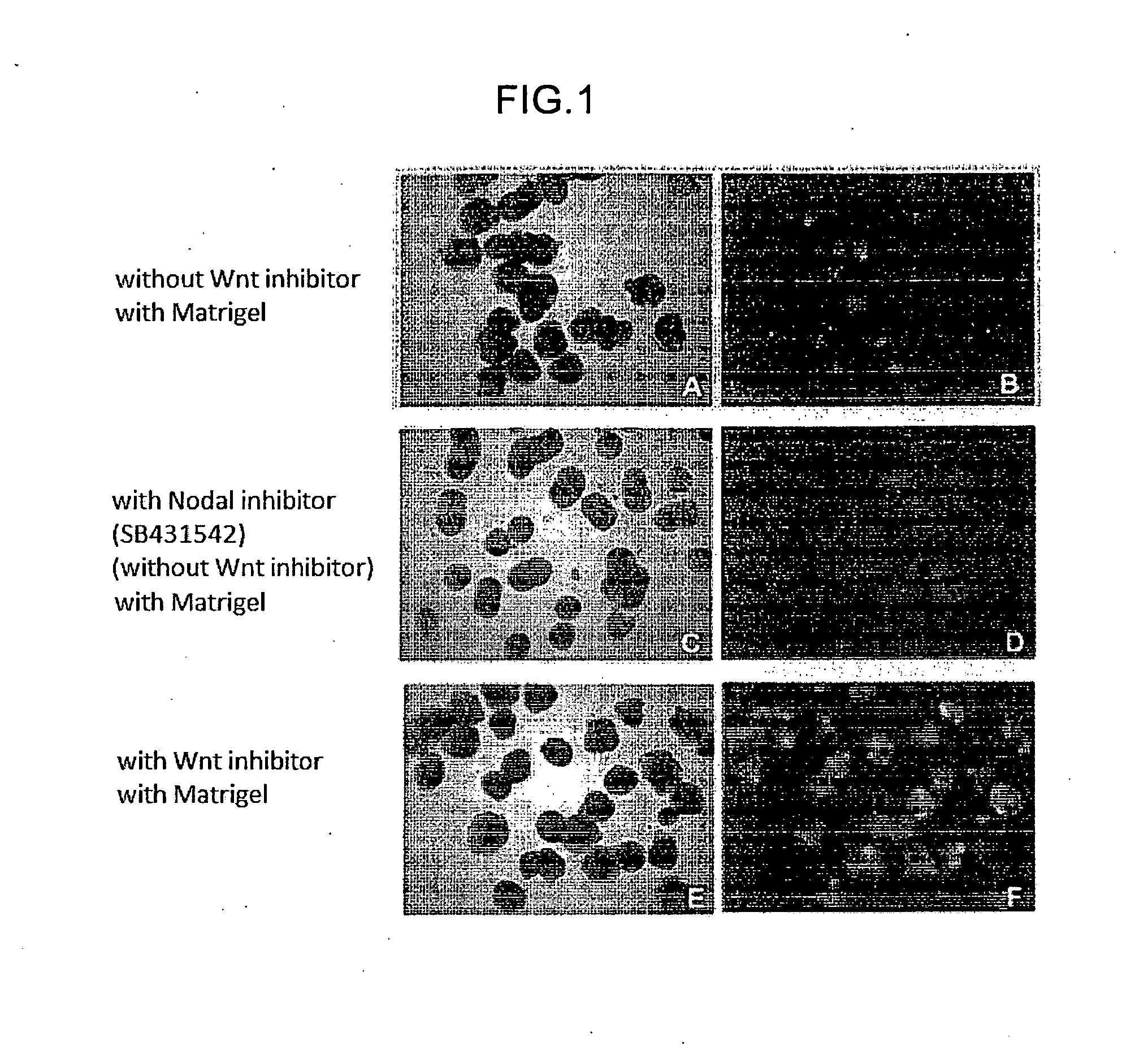
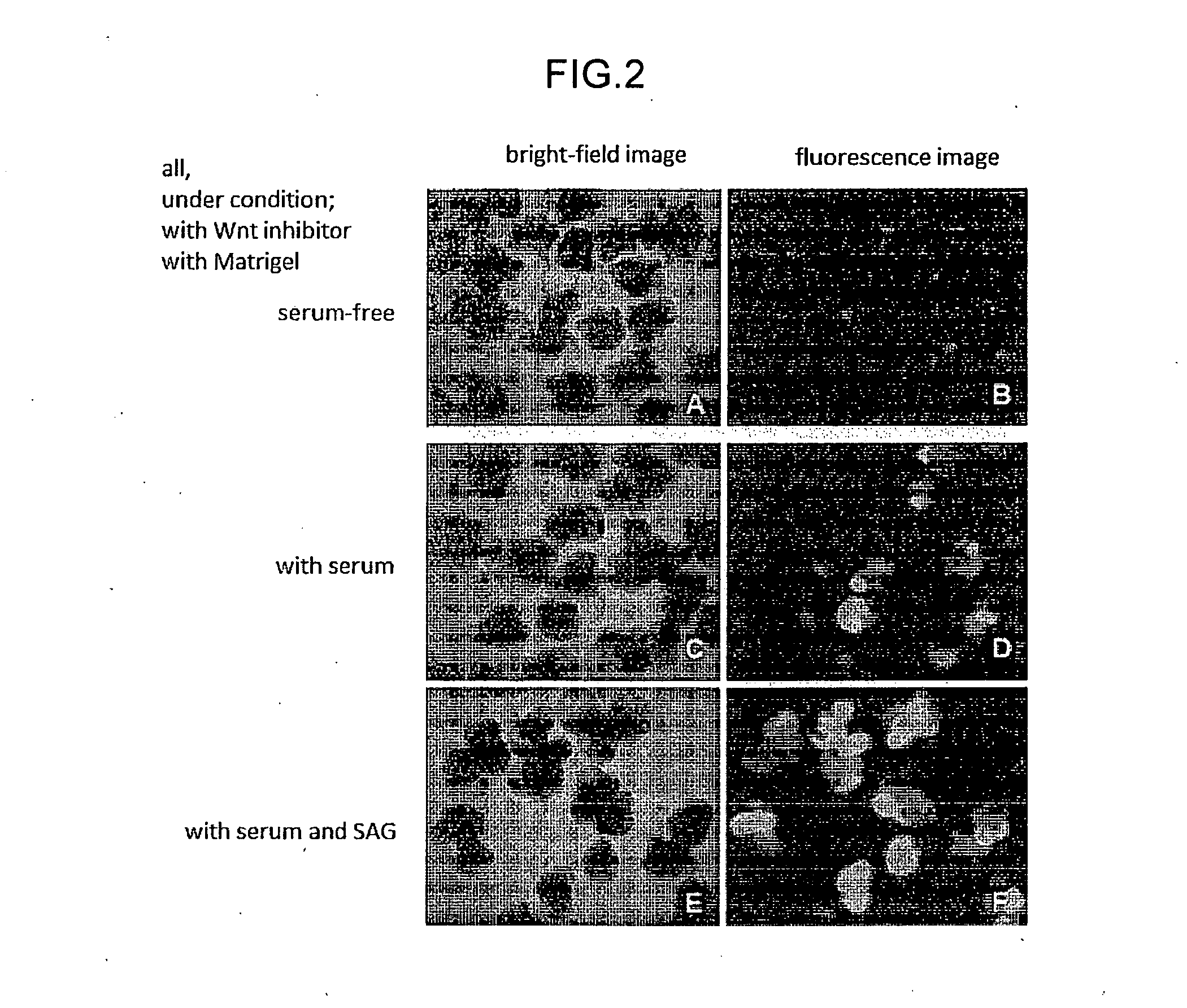
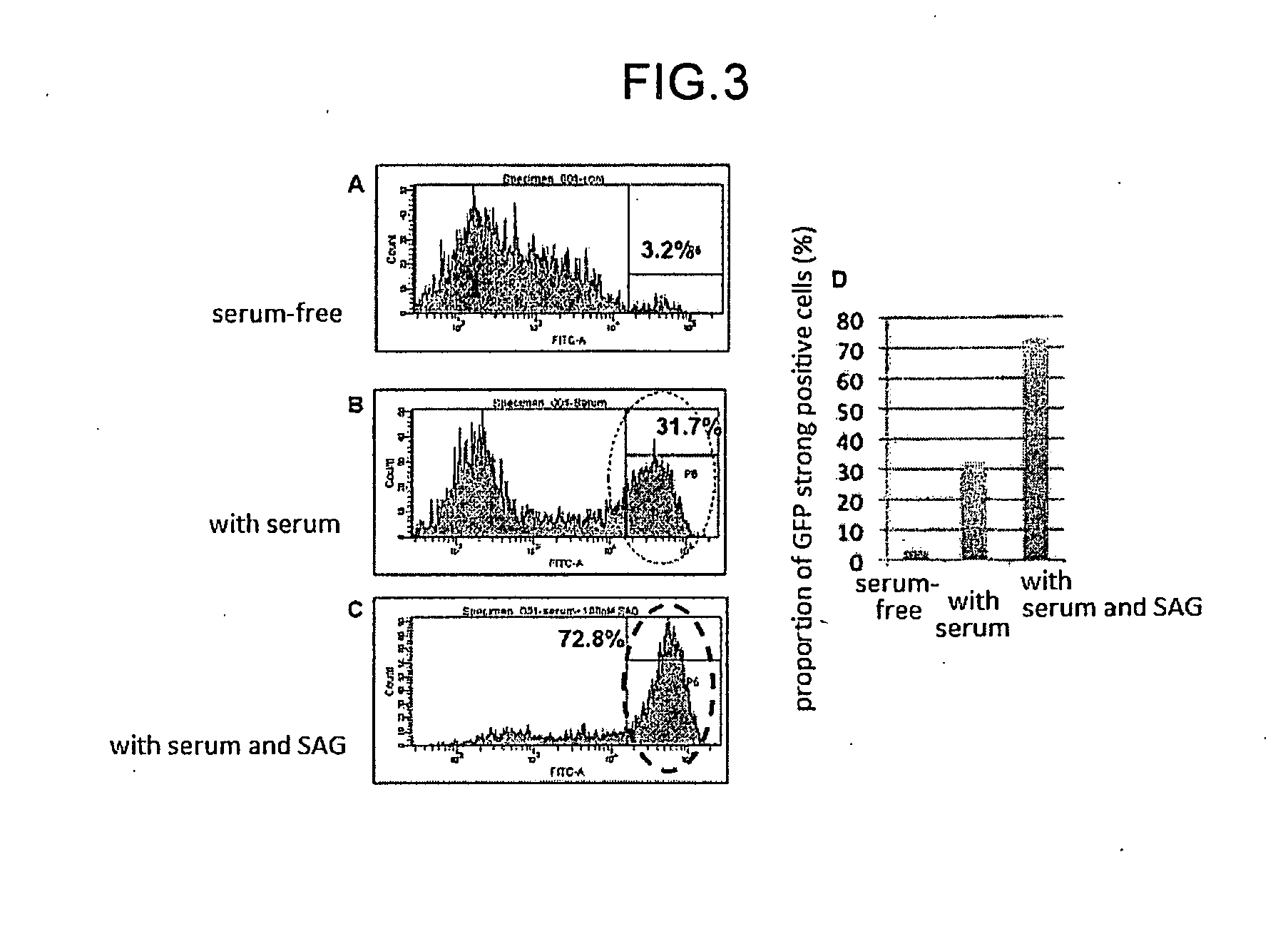
The invention provides a method for producing a retinal tissue by (1) subjecting pluripotent stem cells to floating culture in a serum-free medium containing a substance inhibiting the Wnt signal pathway to form an aggregate of pluripotent stem cells, (2) subjecting the aggregate to floating culture in a serum-free medium containing a basement membrane preparation, and then (3) subjecting the aggregate to floating culture in a serum-containing medium. The invention also provides a method for producing an optic-cup-like structure, a method for producing a retinal pigment epithelium, and a method for producing a retinal layer-specific neural cell.
Owner:SUMITOMO CHEM CO LTD +1
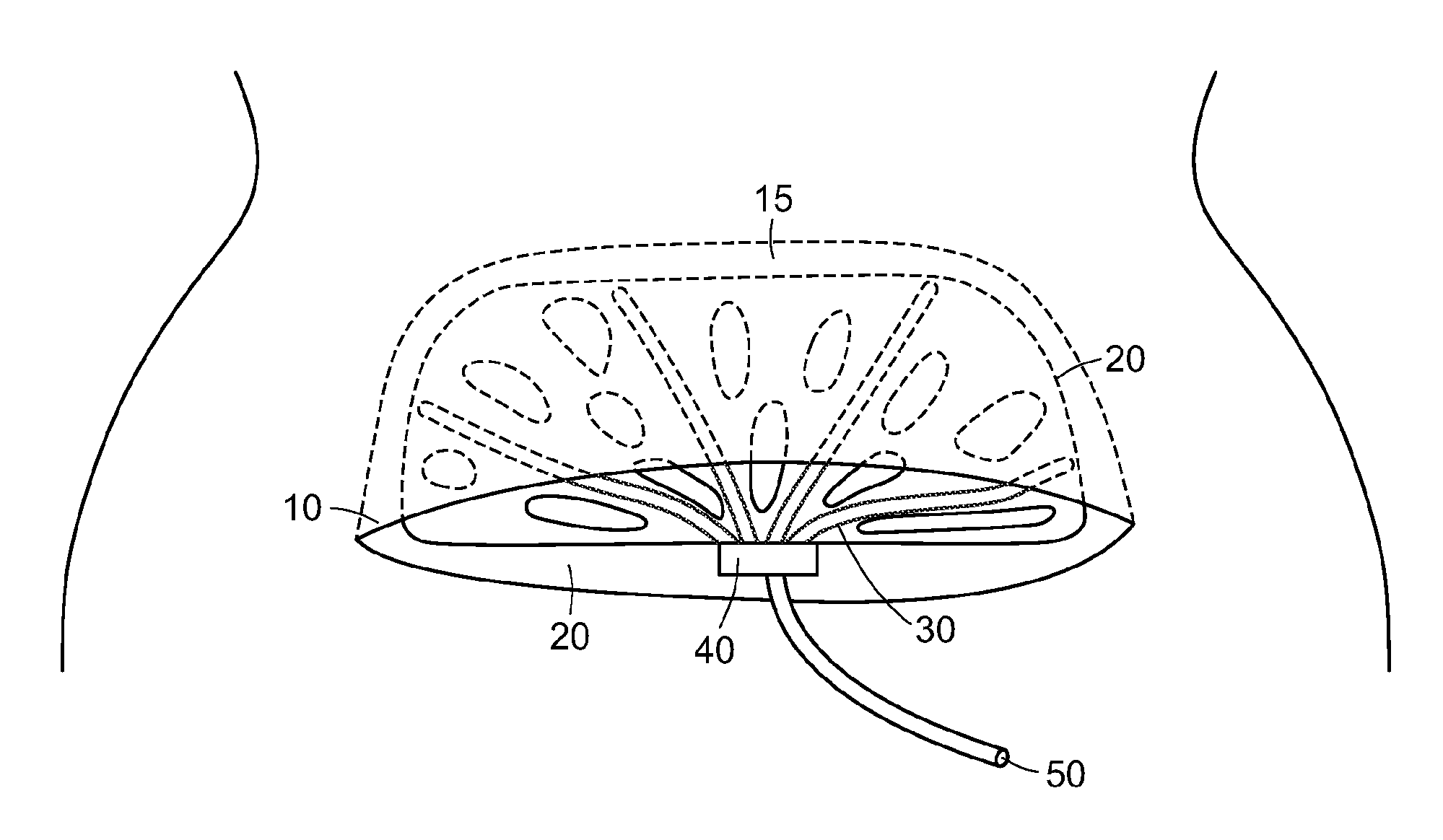
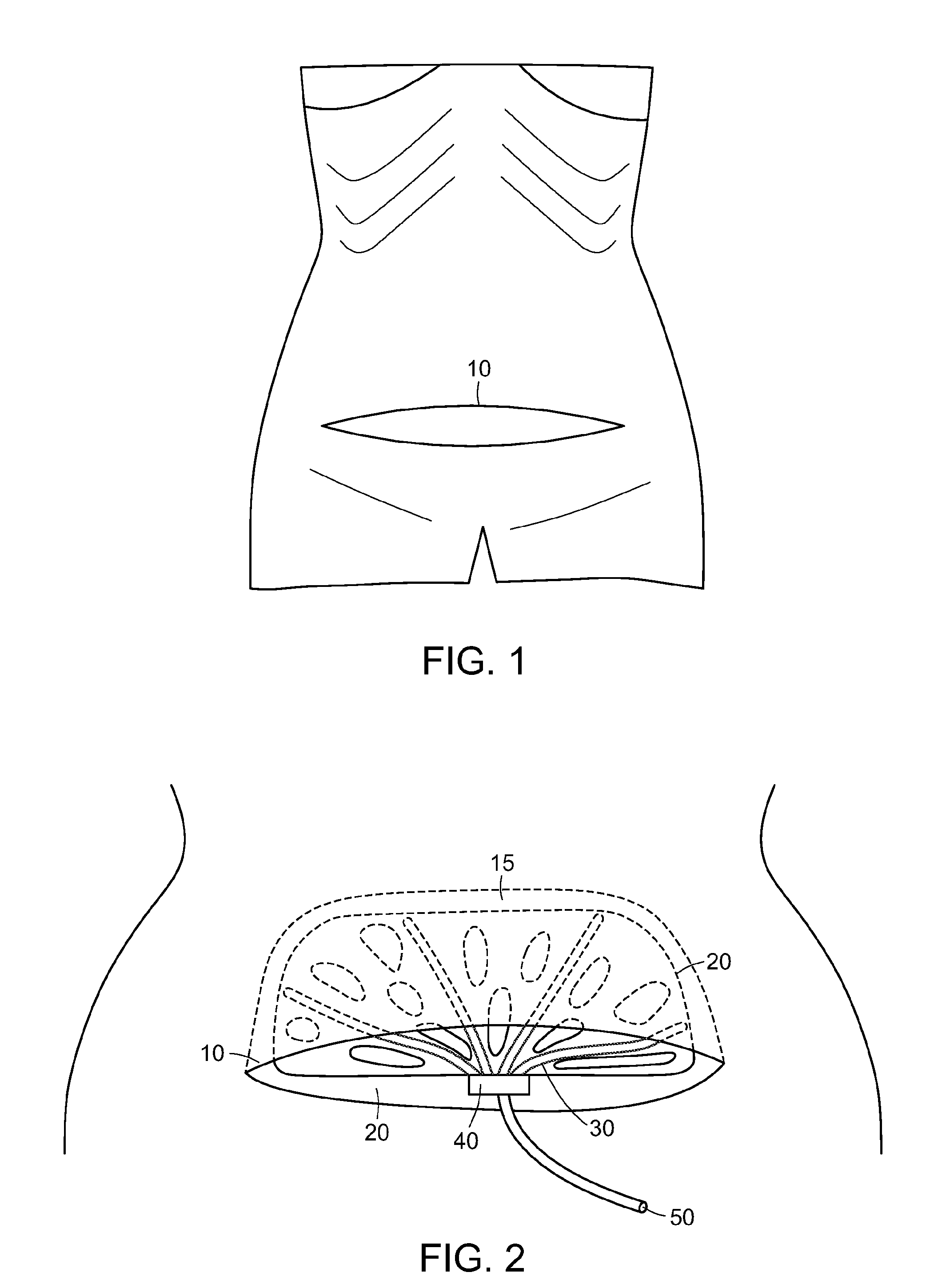
Abdominal postoperative binder and method of use
InactiveUS6270469B1Promote healingDiscourages deep breathingBreast bandagesSuspensory bandagesInjury mouthPeritoneum
The invention is a postoperative binder and method of use. The binder is made of relatively inelastic material that is cut to fit the patient and held in place by a plurality of tails fastened with Velcro(R) binders. The present invention uses mechanical, rather than elastic, compression. Mechanical loads are carried over the iliac crest, by hooking the tails of the binder over the iliac crest and then bifurcating the tails for attachment to the abdominal portion of the binder. The present invention provides support of lower abdominal tissue, especially near the genitals and in the area of the peritoneum. The invention is also a method of preventing post-operative wound infection, reducing incidence of seroma and hematoma formation and wound separation while reducing pain and the need for pain medication by passing a relatively inelastic abdominal binder over the patient's iliac crest and abdomen to place the binder in tension so as to provide a greater than 180 degree radius of compression to the wound.
Owner:MOTT GEORGE E
Specific culture medium for lung tumor organ and stentless 3D culturing method
ActiveCN110592022AStrong cell stemnessRetain heterogeneityCulture processCell culture active agentsY-27632HEPES
The invention discloses a specific culture medium for a lung tumor organ and a stentless 3D culturing method. The specific culture medium is prepared from the following components: FBS, double antibody, N-2, Noggin, B-27, EGF, FGF-10, Y-27632, A 83-01, SB202190, N-acetylcysteine, HEPES, Glutamax, IGF-1, hydrocortisone and Advanced DMEM / F12. The culturing method comprises the following steps: adding a tumor cell into a low serum culture medium, re-suspending the tumor cell, inoculating the tumor cell into a culture vessel, adding the specific culture medium into the culture vessel, changing thespecific culture medium once a day, and performing culturing until an organoid is formed. According to the culture medium and culturing method, a tumor organoid can quickly generate, can be stably cultured for a long time, is regular in spheroid form and has uniform and controllable size, and the heterogeneity of a tumor tissue of a patient can be well maintained in vitro.
Owner:浙江弘瑞医疗科技有限公司
Method for culturing autologous peripheral blood lymphocyte
InactiveCN104371974AInhibitionEnhance killing activityBlood/immune system cellsBiological activationCytokine
The invention relates to a method for culturing an autologous peripheral blood lymphocyte. The method comprises the following steps: (1) separating a mononuclear cell from peripheral blood, resuspending in an X-VIVO15 serum-free culture medium to obtain cell concentration of 1*10<6> / mL, and culturing for 3 days; (2) supplementing the X-VIVO15 serum-free culture medium to 100 mL, adding IL-21*10<3> U / mL, and culturing for 1 day; (3) supplementing the X-VIVO15 serum-free culture medium to 200-240 mL, adding IL-21*10<3> U / mL, and culturing for 3 days; (4) supplementing the X-VIVO15 serum-free culture medium to 1000 mL, adding IL-21*10<3> U / mL, CTLA-4mAb100n g / mL and PD-1mAb100n g / mL; and (5) culturing for 5-7 days to prepare the autologous peripheral blood lymphocyte. The method disclosed by the invention can be used for improving the activation efficiency and amplification efficiency of an effector cell group by adding multiple monoclonal antibodies and cell factors to the X-VIVO15 serum-free culture medium, and can be used for effectively reducing the content of T regulatory cells by covering CTLA-4 and PD-1 molecules of the surfaces of all CIK cells by loading CTLA-4 and PD-1 antibodies in vitro especially, thus further enhancing the killing effect of the CIK cells on tumors.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Establishment method of solitary pulmonary nodule malignancy probability prediction model
ActiveCN107292114AEfficient use ofHigh reference valueSpecial data processing applicationsPulmonary nodeMalignancy
The invention discloses an establishment method of a solitary pulmonary nodule malignancy probability prediction model. The establishment method particularly includes the steps: acquiring basic information of patients and serum tumor marker levels 1-7 days before operation; dividing patient cases into one group with GGO (ground glass opacity) lesion proportion higher than or equal to 50% and another group with GGO lesion proportion lower than 50% according to the GGO lesion proportion and CT (computed tomography) imaging reports of the patients; setting experiment groups and validation groups in each group of cases according to the proportion of 3:1, performing single-factor analysis on relative data of cases of the experiment groups to initially screen independent risk factors; substituting the independent risk factors into multifactor analysis to obtain independent risk factors for judging benign and malignant SPNs (solitary pulmonary nodules); acquiring the SPN malignancy probability prediction model by the aid of Logistic regression; substituting case data of the validation groups into the model, and verifying the case data of the validation groups. The model is simple and easy to use, used indexes can be acquired by the aid of routine examination and are easy to use, and effective intermediate reference information can be provided for further diagnosis and treatment of doctors according to the model.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
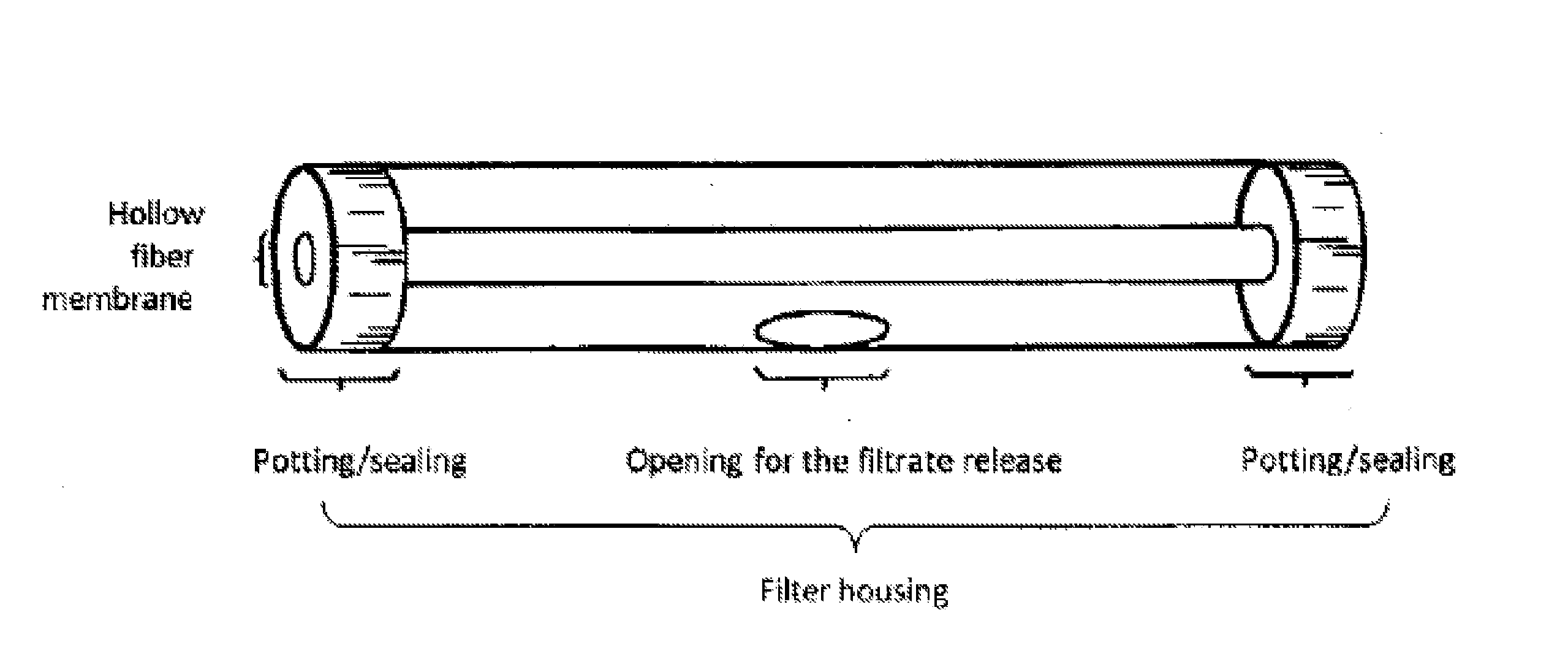
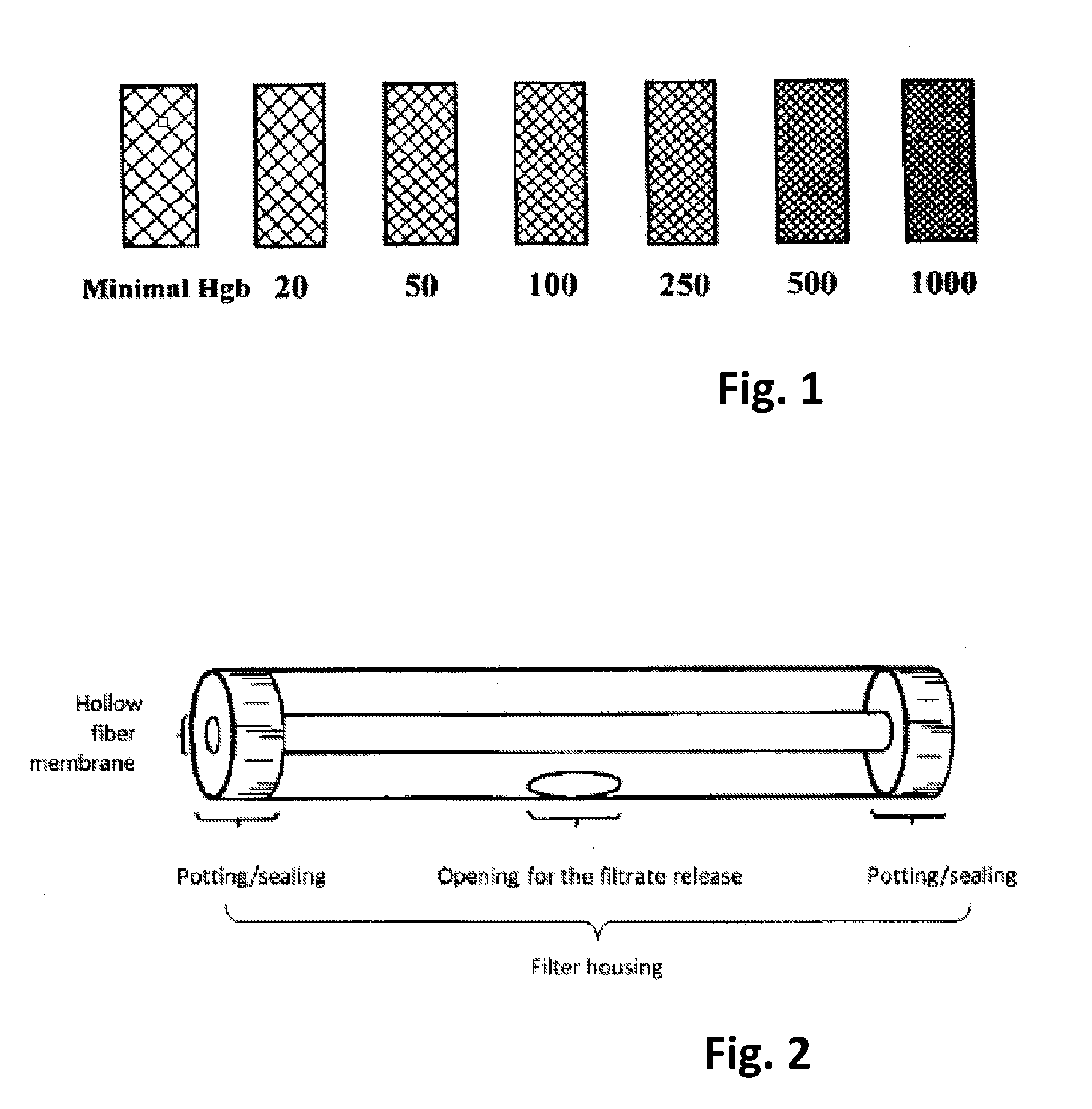
Polymeric Whole Blood Hollow Fiber Membrane Filter Medium and Use Thereof For Separating Blood Plasma/Serum From Whole Blood
ActiveUS20160074569A1Avoid blood leakageAvoid hemolysisMembranesSemi-permeable membranesHemolysisFilter media
A whole blood hollow fiber membrane filter medium is made of a polymeric material having pores of a pore size that ensures permeability to blood plasma or serum but retains blood cells. The whole blood hollow fiber membrane filter medium is used for filtering a whole blood sample so that blood plasma or serum passes through the whole blood hollow fiber membrane filter medium and blood cells are retained. The obtained blood plasma shows no hemolysis.
Owner:MANN HUMMEL GMBH
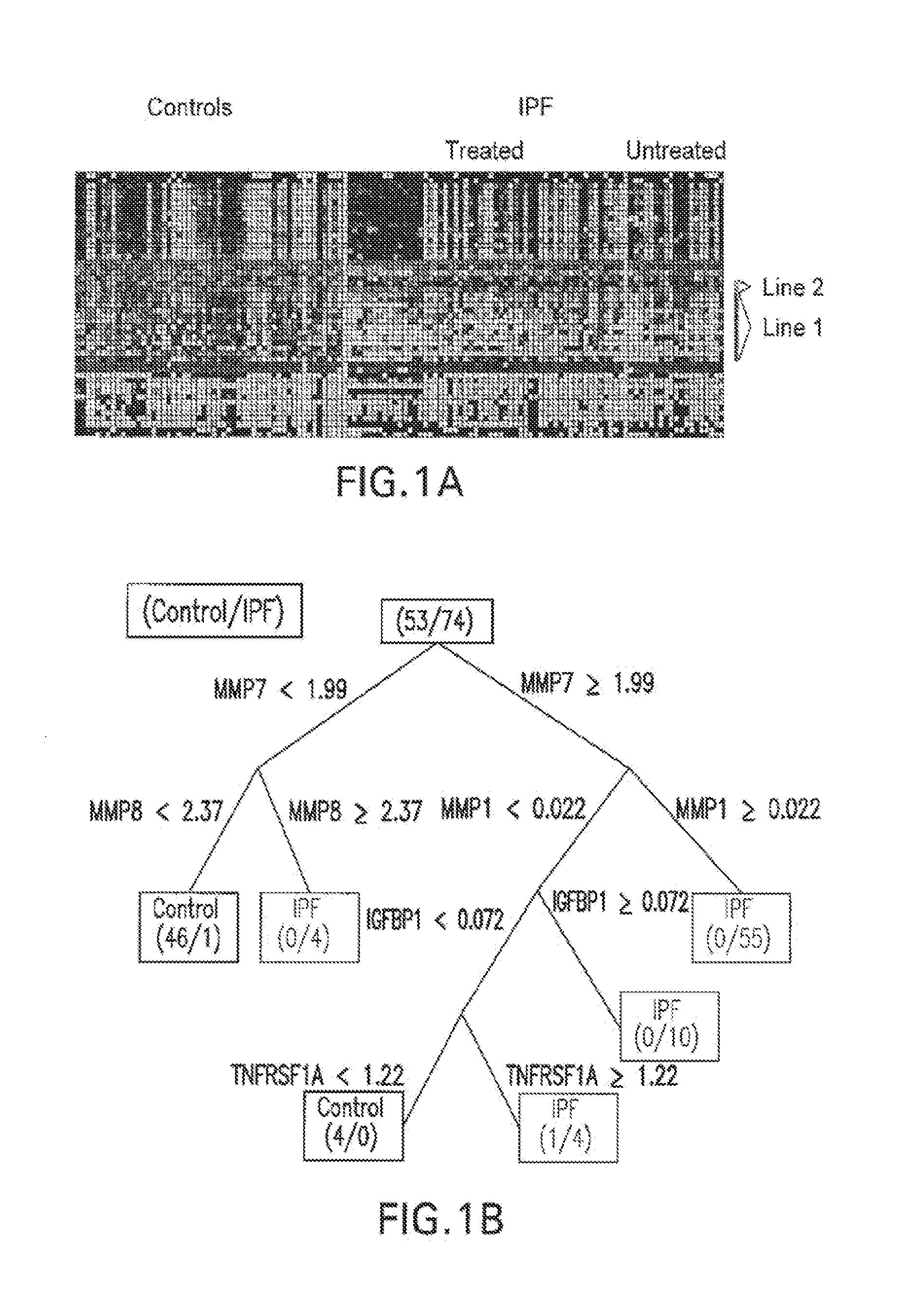
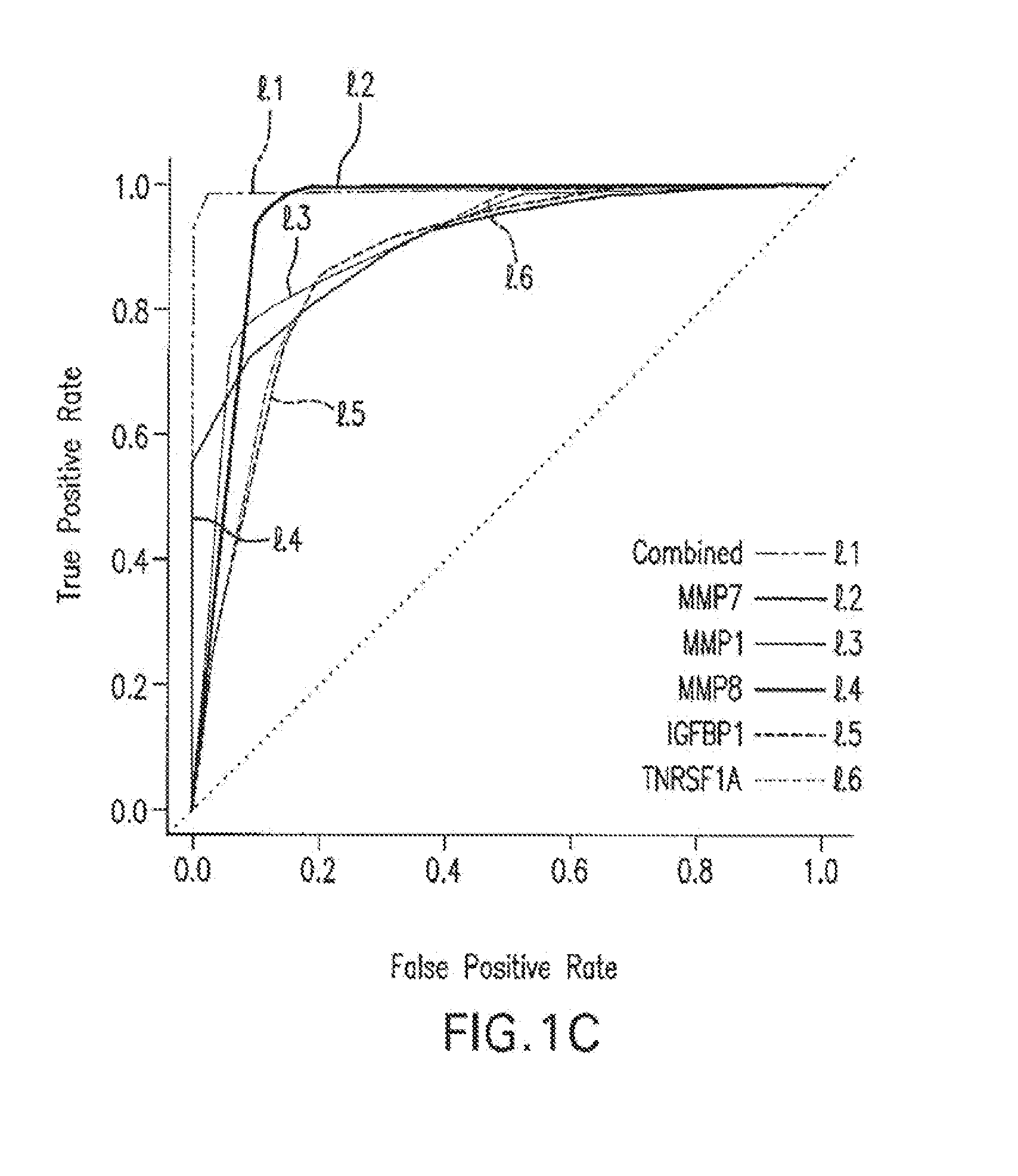
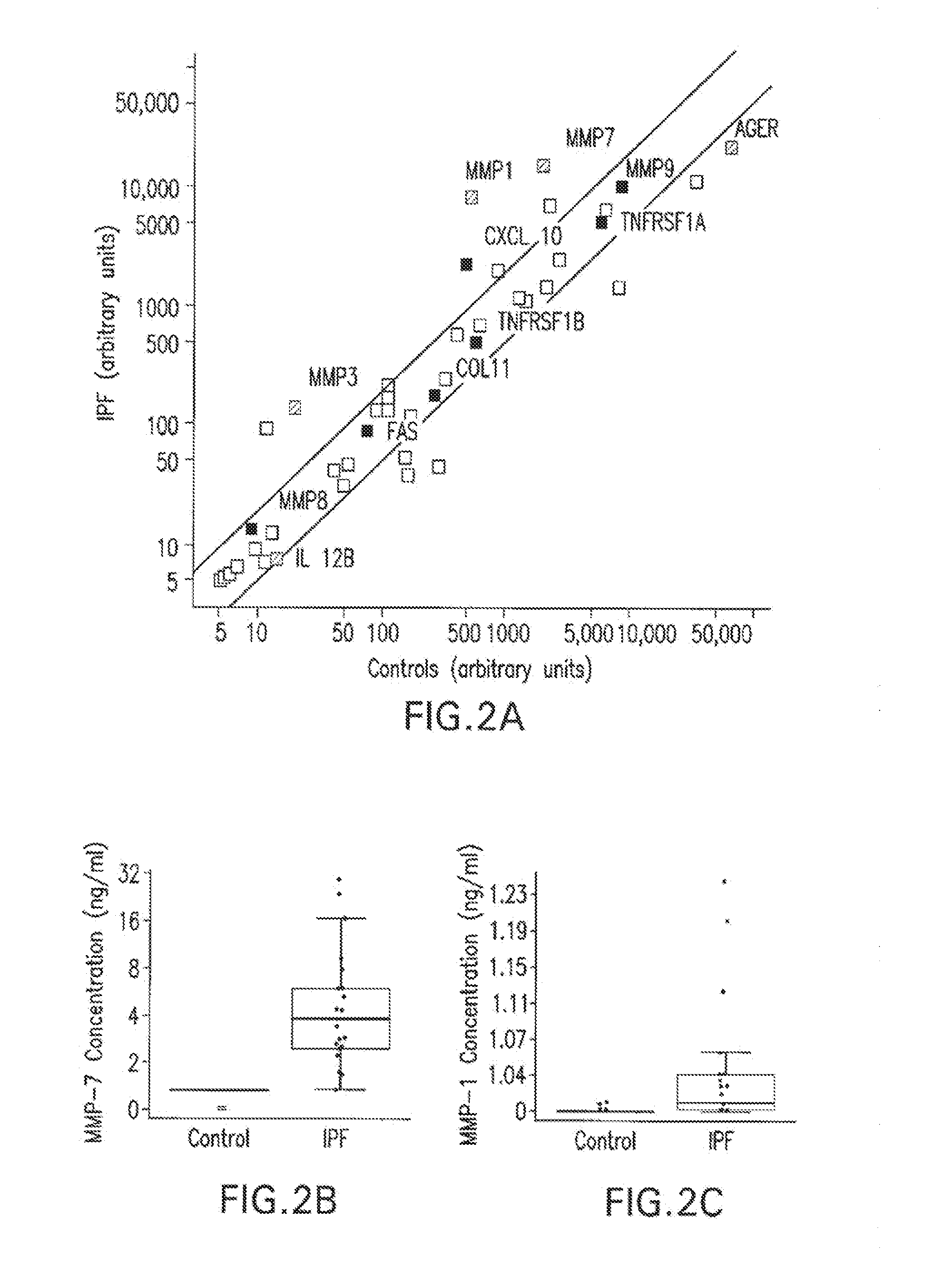
Marker Panels For Idiopathic Pulmonary Fibrosis Diagnosis And Evaluation
ActiveUS20120035067A1Peptide librariesNucleotide librariesIdiopathic pulmonary fibrosisDisease progression
The present invention relates to the discovery that of a panel of serum or plasma markers may be used to diagnose Idiopathic Pulmonary Fibrosis (“IPF”) and distinguish this condition from other lung ailments. It further relates to the identification of markers associated with IPF disease progression.
Owner:UNIVERSITY OF CHICAGO +3
Method for separating and culturing umbilical cord blood mesenchymal stem cells
ActiveCN104630144AIncrease growth rateHigh recovery rateSkeletal/connective tissue cellsPrimary cellBottle
The invention provides a method for separating and culturing umbilical cord blood mesenchymal stem cells. The method comprises the steps of carrying out secondary separation on umbilical cord blood mononuclear cells by using a separating medium, and then inoculating the umbilical cord blood mononuclear cells into a culture bottle in which fibronectin and CD90 monoclonal antibodies are coated; and culturing for 4-5 days by using a serum-free culture system and simulating the in-vivo low-oxygen growth environment of the mesenchymal stem cells, then, removing suspension cells, further culturing adherent cells, and subculturing after the fusion rate of primary cells is up to 60%. By using the method for separating and culturing umbilical cord blood mesenchymal stem cells, provided by the invention, the problems of adherence infirmness, low culture success rate, low purity and the like caused in the extraction and culture processes of the umbilical cord blood mesenchymal stem cells are effectively solved, and the safety in clinical application is improved. In addition, the application ranges of the umbilical cord blood mesenchymal stem cells are widened, the utilization values of the umbilical cord blood mesenchymal stem cells are developed, and the clinical application prospects of the umbilical cord blood mesenchymal stem cells are widened.
Owner:中国医科大学 +1
Surgical cavity drainage and closure system
ActiveUS20140039468A1Promote rapid healing and stabilizationEasy adhesionSuture equipmentsSurgical needlesSurgical departmentBiodegradable polymer
A surgical drain device includes an adhesion matrix of biodegradable polymer material and a plurality of drain tubes attached to the matrix. The device is implanted within a surgical wound to treat the presence of seromas, for example, and is used to promote drainage, tissue adhesion, and wound closure. The drain tubes converge into a common collection tube that leads wound fluid outside the body under gravity feed or negative pressure applied to the collection tube. The matrix contains an array of apertures that allow tissue contact across the device. The device also can include a coating of surgical adhesive and a tissue anchoring system of hooks or barbs. The device and systems containing the device are particularly useful to promote the healing of surgical wounds from abdominal surgery.
Owner:UNIV OF MASSACHUSETTS
Method for preparing corneal endothelial cell
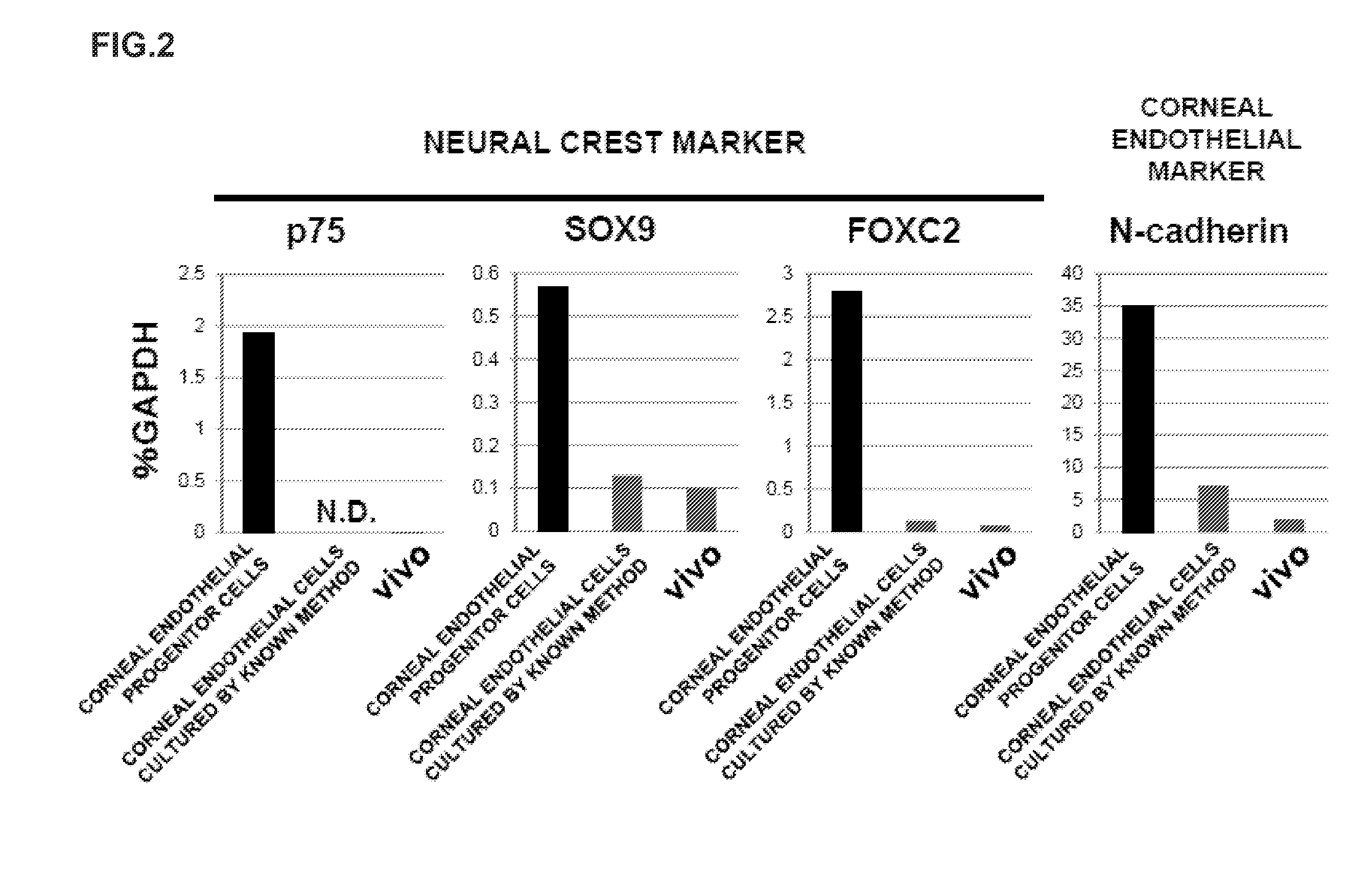
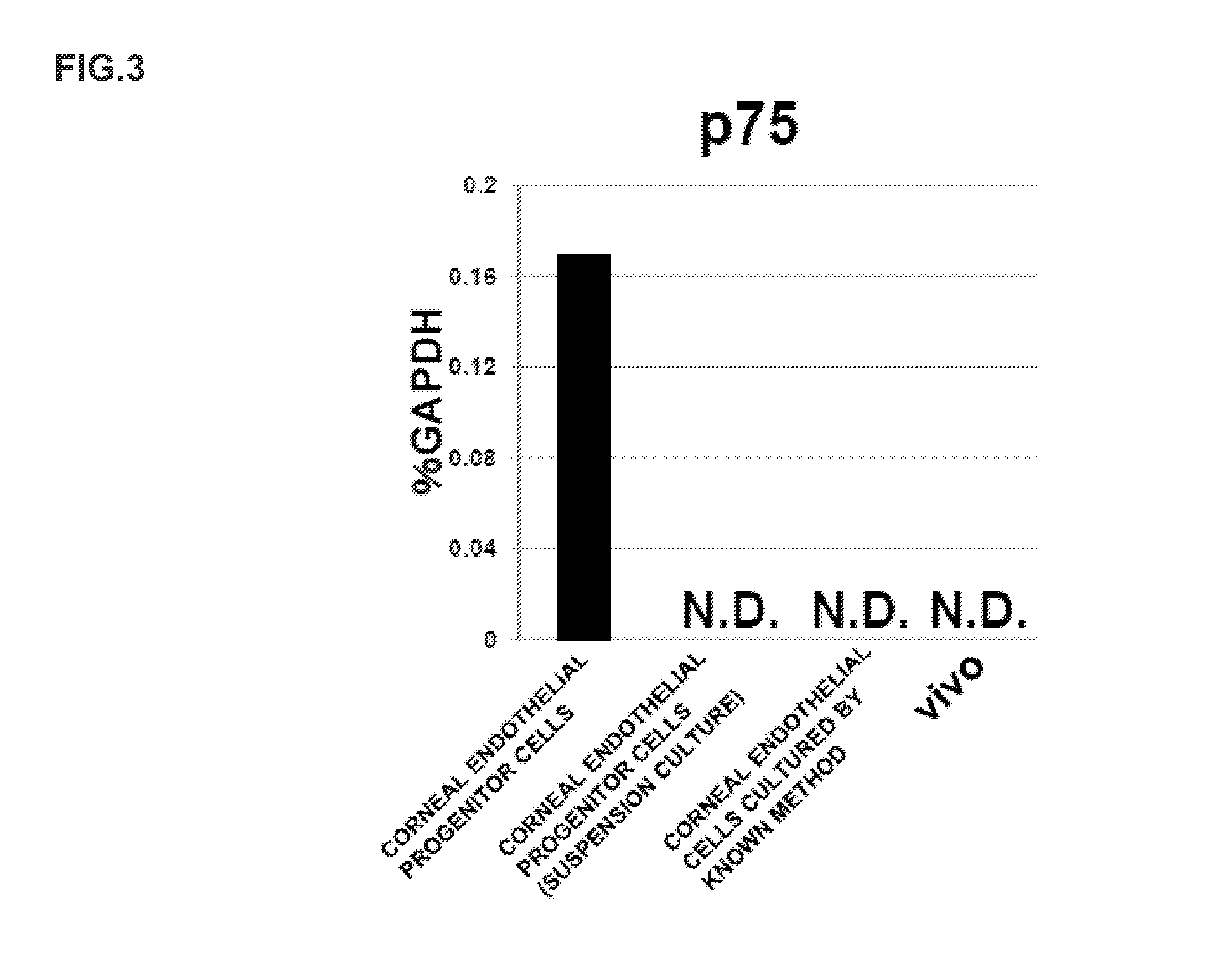
ActiveUS20140170751A1Improve proliferative abilityIncrease the number ofNervous system cellsUnknown materialsDiseaseProgenitor
The present invention relates to a method for preparing corneal endothelial progenitor cells by adherent culturing a cell population isolated from corneal endothelial cell tissue at a low density by using a serum-free medium. The present invention also relates to a method for preparing corneal endothelial cells by differentiation-inducing the corneal endothelial progenitor cells obtained by the aforementioned method. According to the present invention, corneal endothelial progenitor cells can be selectively grown from a corneal tissue-derived cell population, and corneal endothelial cells obtained by inducing the corneal endothelial progenitor cells can be applied to treatment of corneal endothelial diseases. As a result, problems of corneal transplantation such as shortage of donors and occurrence of rejection can be solved.
Owner:OSAKA UNIV
Method of inducing and differentiating human umbilical cord derived mesenchymal stem cells into cartilage cells
The invention relates to a method of inducing and differentiating human umbilical cord derived mesenchymal stem cells into cartilage cells. The method includes: in order to solve the problem that the prior art is low in differentiation efficiency, cleaning umbilical cord tissue to remove blood stain, cutting the same into small sections, dissecting the small sections to remove vascular tissue, cutting the small sections into pieces, and digesting to obtain a single mesenchymal stem cell; culturing the obtained mesenchymal stem cell in an amplification manner to the seventh generation to obtain a unicellular suspension, inoculating the unicellular suspension into a serum-free culture medium containing a cartilage differentiation inducer to continue culturing, replacing a fresh culture medium every other 3.5 days, and inducing for 14 days to obtain the cartilage cells. The method can efficiently differentiate the umbilical cord mesenchymal stem cells into the cartilage cells and is of important significance in the aspects of restoring cartilage tissue in human bodies and treating bone injury.
Owner:中卫华医(北京)生物科技有限公司 +1
Method for culturing autologous peripheral blood lymphocytes
ActiveCN102816735AImprove activation efficiencyImprove efficiencyBlood/immune system cellsPeripheral blood mononuclear cellEffector cell
The invention relates to a method for culturing autologous peripheral blood lymphocytes, comprising the following steps of: (1) separating a PBMC (Peripheral Blood Mononuclear Cell) from peripheral blood, then heavily suspending in a serum-free culture medium, and statically culturing to prepare a primary culture solution; (2) replenishing the serum-free culture medium to the primary culture solution, simultaneously adding IL (Interleukin)-2, and statically culturing to prepare a secondary culture solution; (3) replenishing the serum-free culture medium to the secondary culture solution, simultaneously adding IL-2, and statically culturing to prepare a culture solution; and (4) uniformly dividing the culture solution into two parts, adding the serum-free culture medium to complement a sufficient volume, adding IL-2, statically culturing, and repeating the step (4) to prepare the autologous peripheral blood lymphocytes. As multiple monoclonal antibodies and cell factors are added to the serum-free culture medium, the activation efficiency and the amplification efficiency of effector cell masses are improved. Through detection, the activation efficiency and the amplification efficiency are obviously improved as compared with the existing method, and a large amount of cell masses occur after the effector cell masses are activated about 3 days.
Owner:山东省齐鲁细胞治疗工程技术有限公司
Freezing storage protective solution for human umbilical Wharton's jelly tissue block
InactiveCN101971799AGuaranteed osmotic balanceLow toxicityDead animal preservationSucroseCell membrane
The invention discloses freezing storage protective solution for a human umbilical Wharton's jelly tissue block. 1 liter of the freezing storage protective solution comprises 0.8 to 1.2 mol of glycol, 0.4 to 0.6 mol of dimethyl sulfoxide, 0.1 to 0.3 mol of cane sugar, 100 to 200 milliliters of cord blood autoserum and the balance of culture solution DMEM / F12. In the freezing storage protective solution, the cord blood autoserum and the culture solution DMEM / F12 serve as base solution to provide nutrient for the tissue block, so osmotic equilibrium inside and outside cells is guaranteed, the cell environment in the tissue block is guaranteed to be similar to the in vivo environment, and the freezing storage effect is good; the low-concentration glycol and dimethyl sulfoxide serve as a permeability freezing protective agent, so the toxicity of the freezing storage protective solution to the cells is reduced; and the cane sugar serves as a non-permeability freezing protective agent, so the viscosity of the solution is increased and the osmotic pressure inside and outside cell membranes is relieved when the cells are frozen and stored. The human umbilical Wharton's jelly tissue block can be directly frozen and stored by the freezing storage protective solution, so the freezing storage effect is good, the cell damage is little, and high-quality human umbilical mesenchymal stem cells can be provided for clinic.
Owner:江苏省北科生物科技有限公司
Portable device with disposable reservoir for collection of internal fluid after surgery
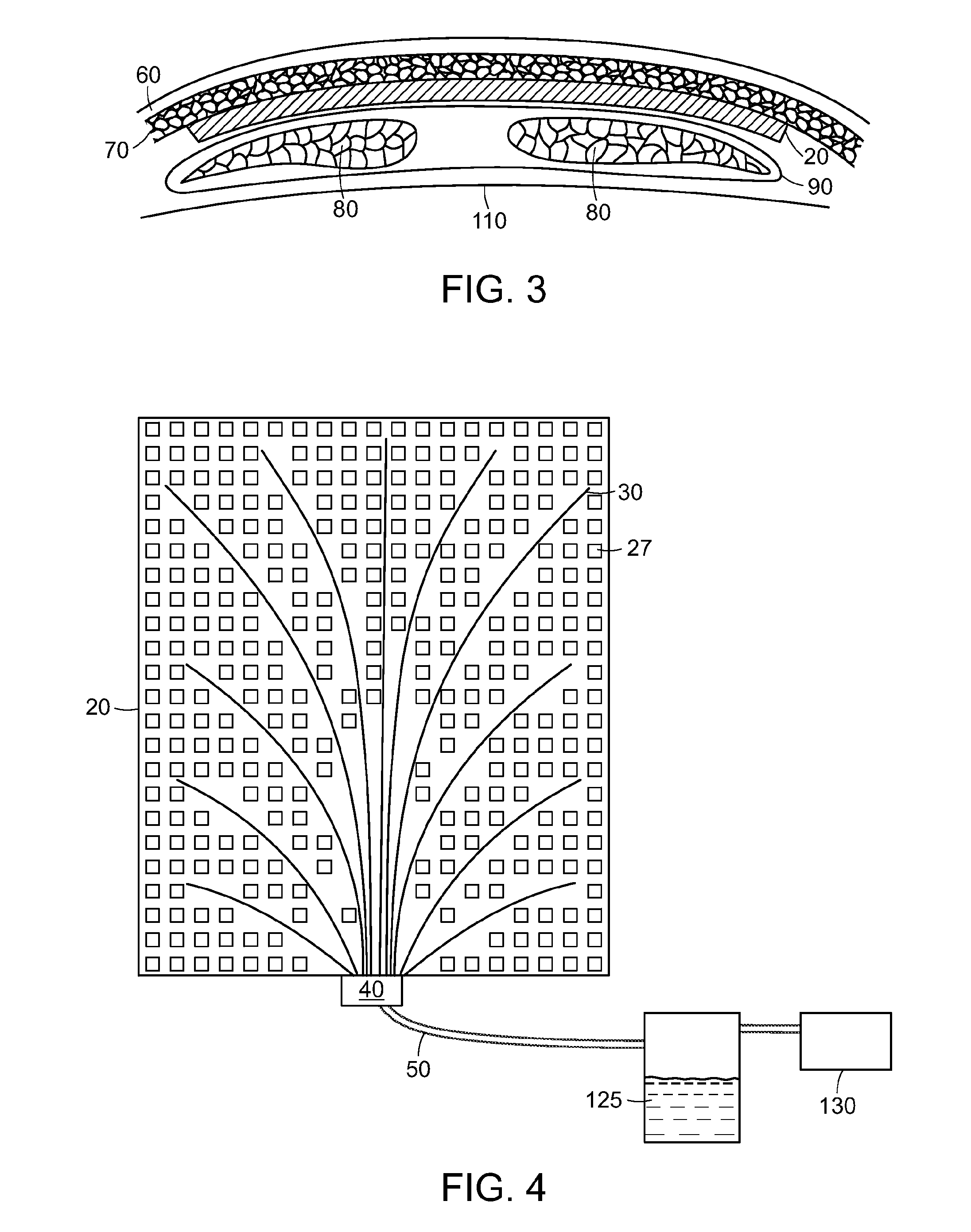
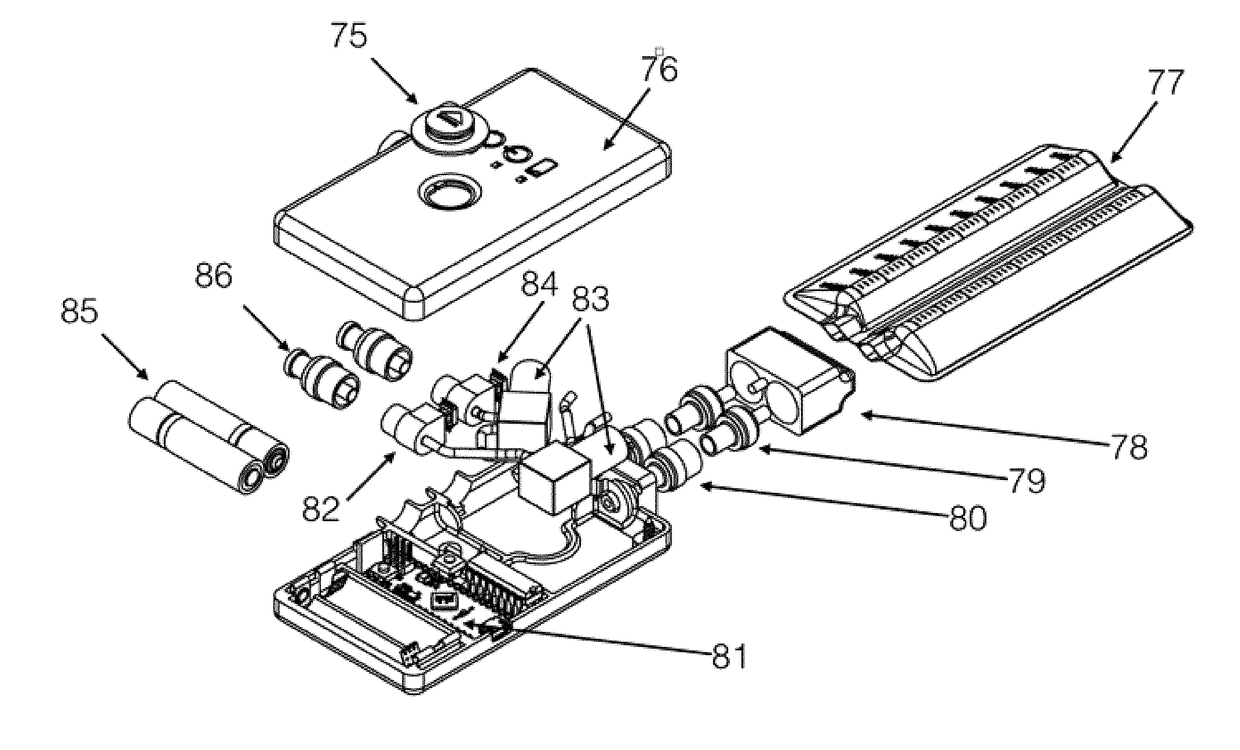
ActiveUS20180001000A1Easy to handleImprove sealingFlexible member pumpsMedical devicesSerosanguinous fluidSeroma
A system and apparatus for the collection of serous or serosanguinous fluid from a percutaneous site after surgery. A pump unit with one or more pumps or powered sources provide continuous negative pressure suction to draw fluid from the percutaneous site and pumps the fluid into disposable reservoirs with one-way valves that are easy to handle while maintaining sterility and a seal to prevent the loss of vacuum. Air is continuously removed from the reservoirs. Measurement and analysis of the output is performed automatically.
Owner:SOMAVAC MEDICAL SOLUTIONS INC
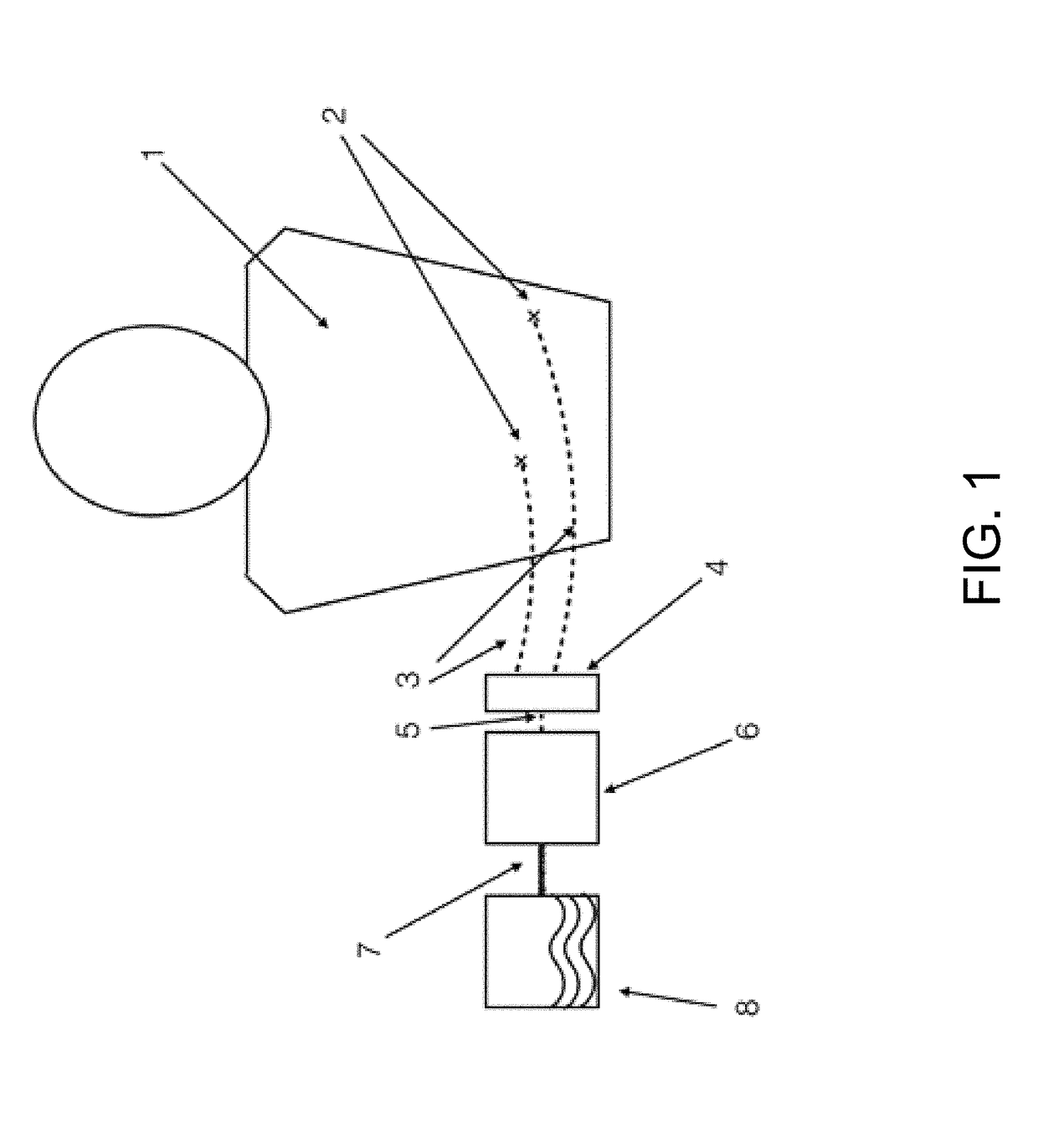
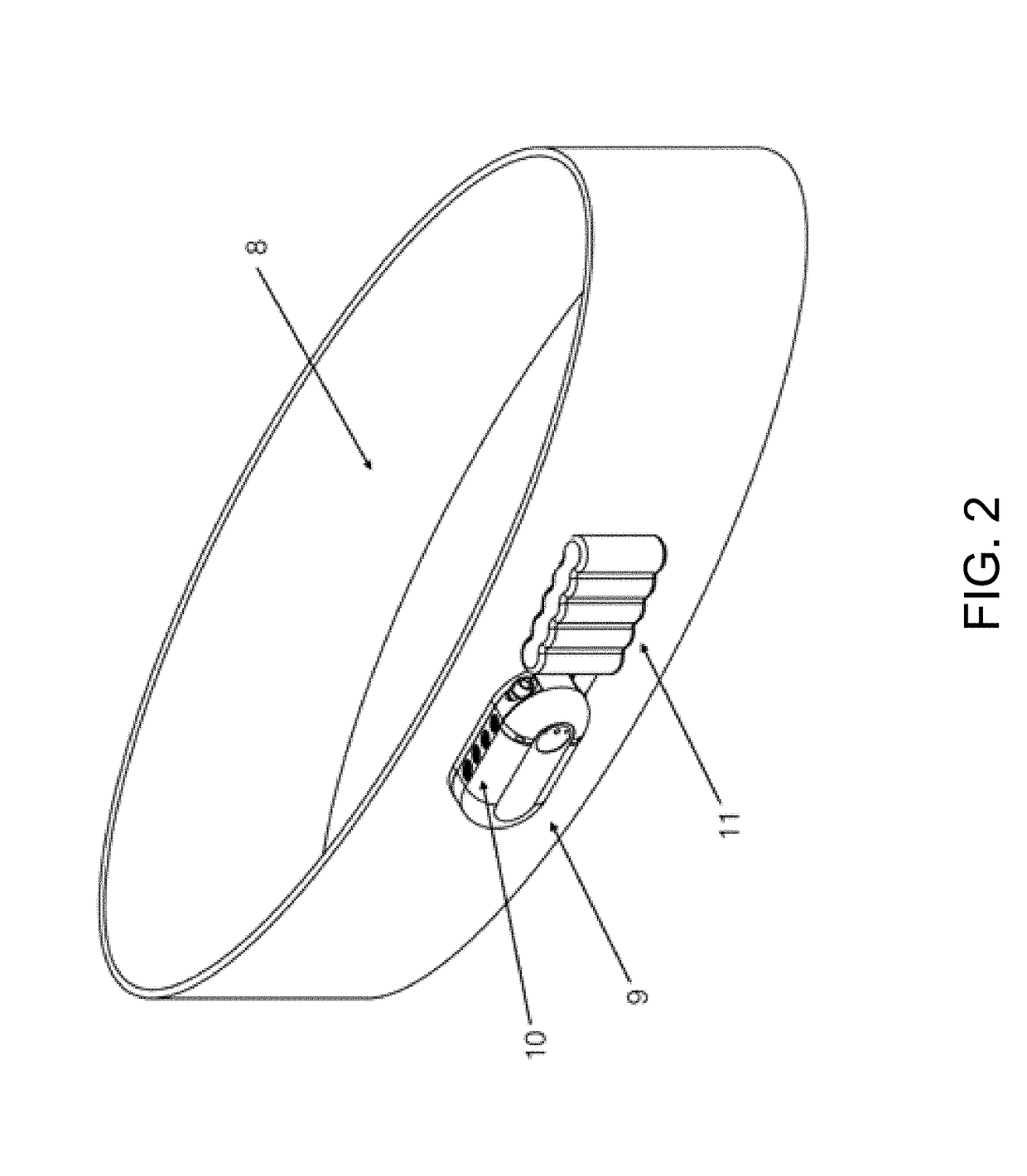
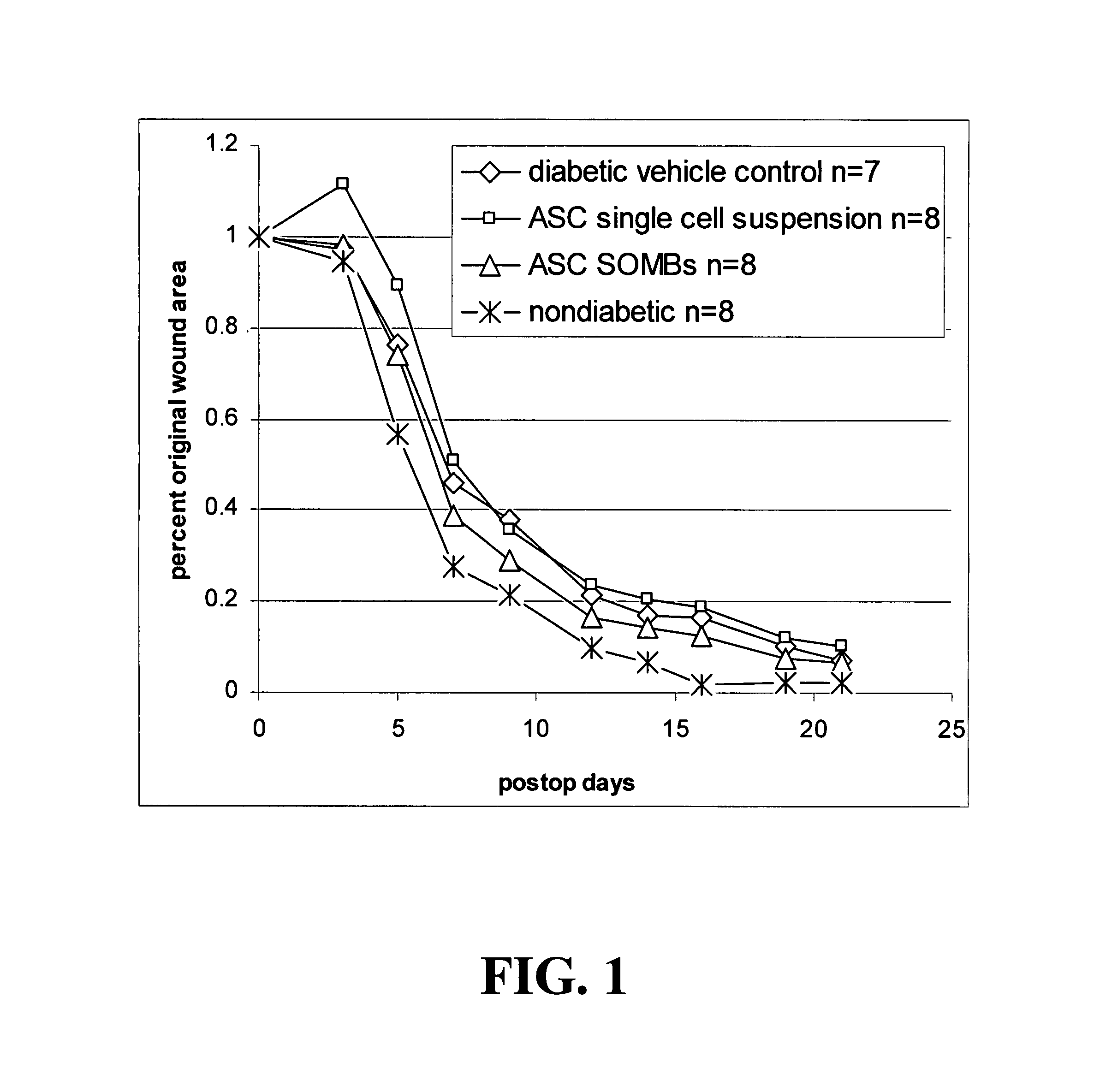
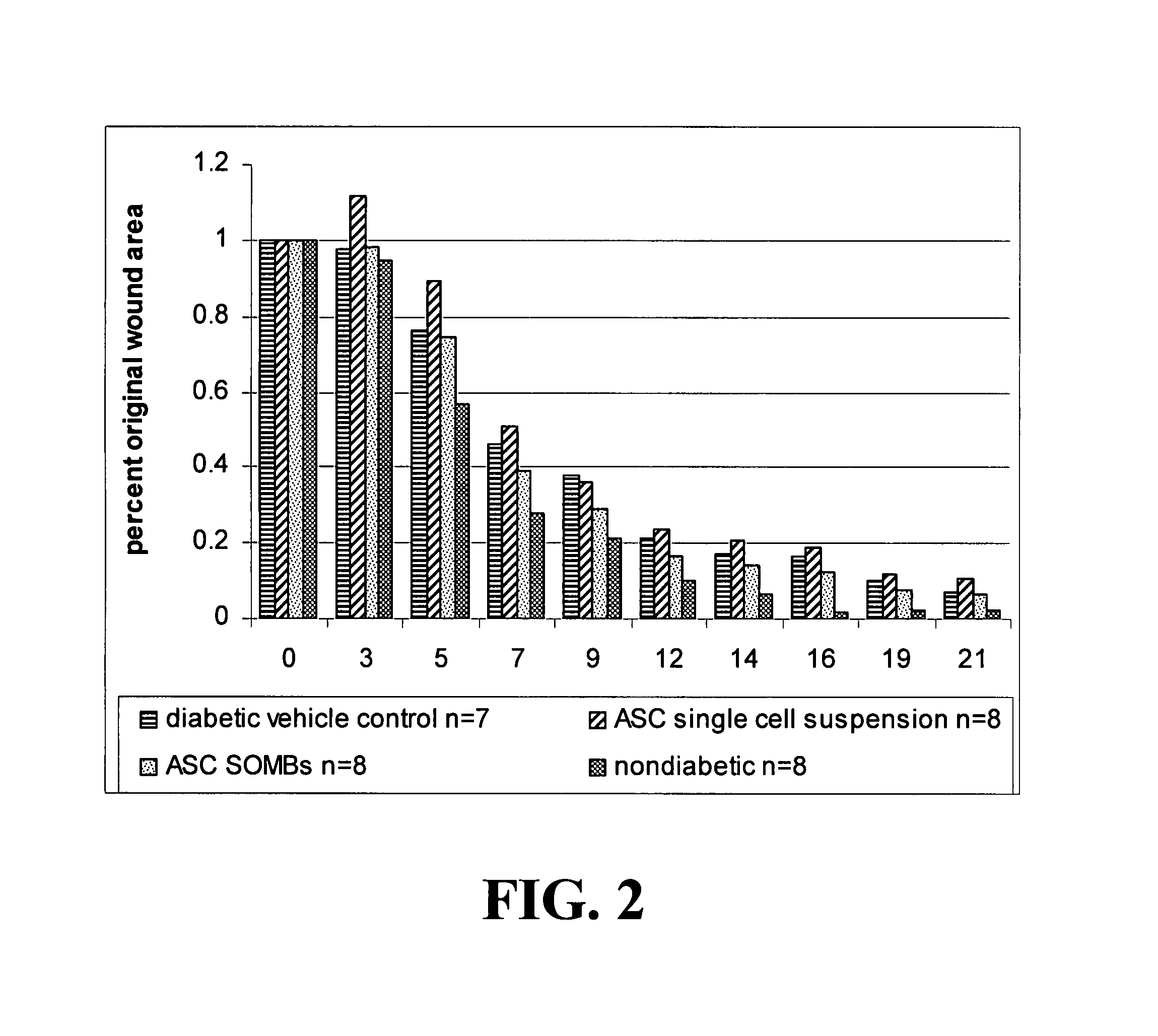
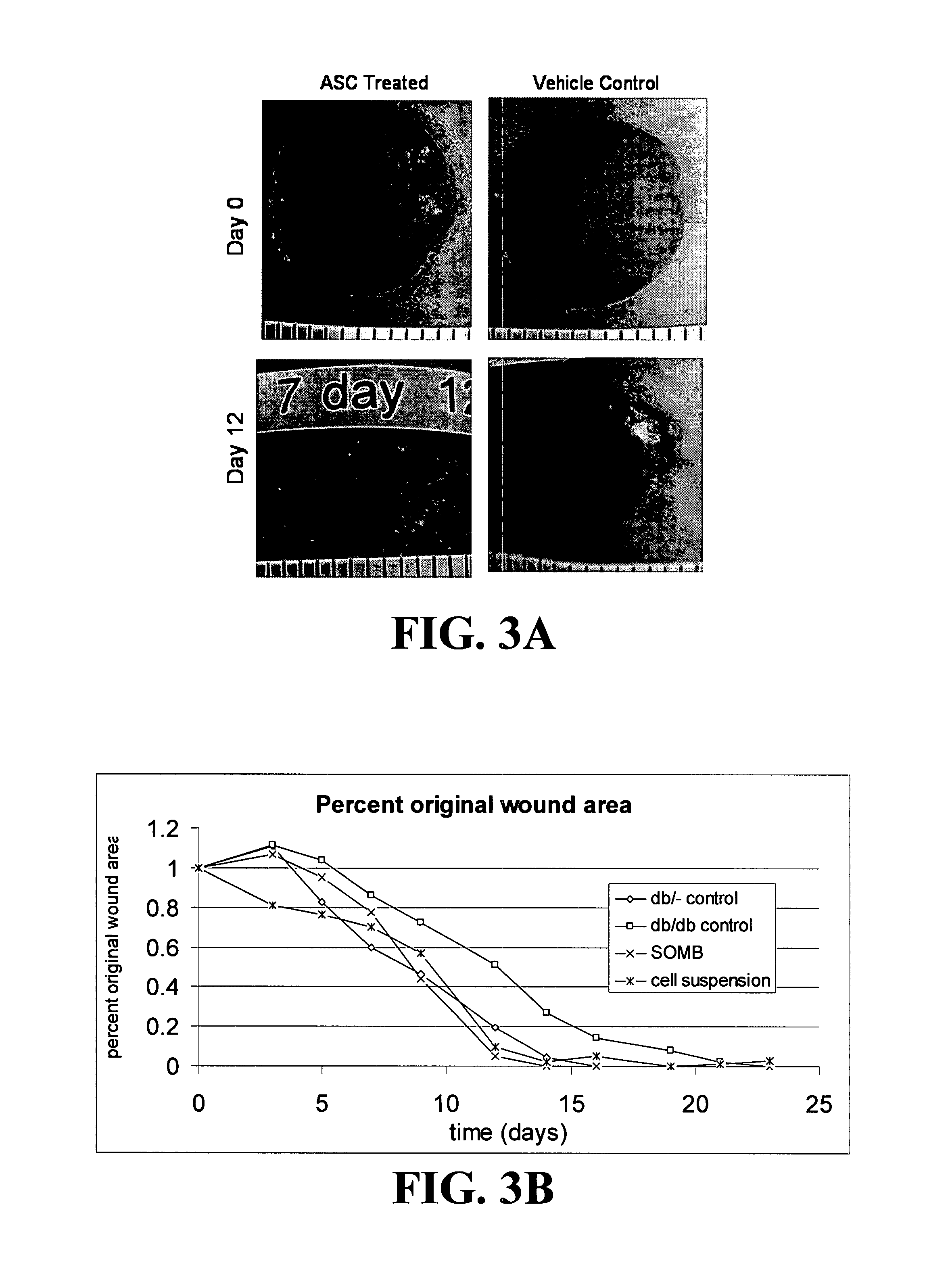
Methods and compositions useful for diabetic wound healing
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Formation of neuromuscular junctions in a defined system
A method for forming neuromuscular junctions includes forming functional neuromuscular junctions between motoneurons and muscle cells by co-culturing one or more human motoneurons and one or more rat muscle cells in a substantially serum-free medium. A synthetic mammalian neuromuscular junction includes a human motoneuron functionally linked to a rat muscle cell in a substantially serum-free medium. An artificial substrate may be used to support the one or more neuromuscular junctions.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Culture medium for keeping primary airway epithelial cells in physiological state in vivo
InactiveCN102505005AStrong growthEasy to prepareArtificial cell constructsVertebrate cellsPhysiologyBovine serum albumin
The invention relates to preservation of human local living parts and in particular relates to a culture medium for keeping primary airway epithelial cells in the physiological state in vivo during in vitro culture. The invention is characterized in that the culture medium contains the following components (per liter), 12 g of DMEM / F12 medium, 30 mg of penicillin G sodium salt, 50 mg of streptomycin, 10 mg of insulin, 5 mg of transferring, 25 Mug of epidermal growth factors, 100 Mug of cholera toxin, 108.741 Mug of hydrocortisone, 1 g of bovine pituitary extract, 3 g of bovine serum albumin, 1.5*10<-2> Mug of retinoic acid, 50 mg of gentamycin, 0.5 mg of amphotericin B, 50 mL of fetal calf serum and the balance of water. The primary airway epithelial cells cultured by the culture medium have the ciliary beating function and can maintain the physiological state in vivo.
Owner:SOUTHERN MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com