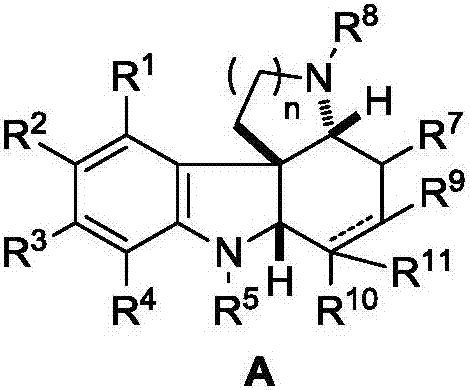
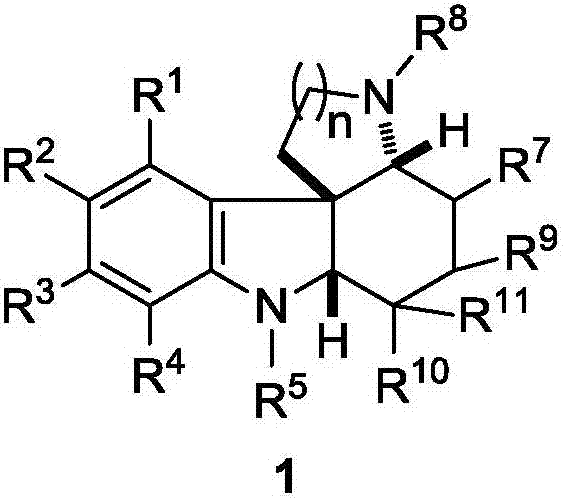
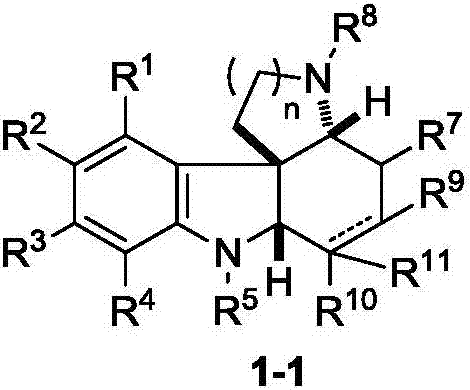
Fused polycyclic indoline compound and preparation method thereof as well as pharmaceutical composition and application
A polycyclic indoline compound technology, applied in the field of fused polycyclic indoline compounds, can solve the problems of long synthesis steps, cumbersome operations, and inability to apply industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Enamine 14a (0.3mmol), indium trichloride (3.3mg), Molecular sieves (200 mg), and 2 mL of dichloromethane were added to the reaction tube, and then dimethyl methylene malonate (R 9 =H, 0.9 mmol) in 2 mL of dichloromethane, reacted at room temperature for 5 hours to obtain product 1a, 134.9 mg, yield 90%, 95 / 5dr. 1 H NMR (400MHz, CDCl 3 )δ7.66(d,J=8.0Hz,2H),7.39–7.35(m,3H),7.13(td,J=8.0,1.2Hz,1H),6.73(td,J=7.2,0.8Hz,1H ),6.51(d,J=8.0Hz,1H),4.28(d,J=1.2Hz,1H),3.70–3.63(m,7H),3.34(t,J=10.0Hz,1H),3.13–3.09 (m,1H),2.82(s,3H),2.46(s,3H),2.20–2.11(m,2H),1.89–1.85(m,2H),1.79–1.75(m,1H),1.69–1.61 (m,1H).
[0113]
Embodiment 2
[0115] Enamine 14b (0.3mmol), ferric chloride (3.3mg), Molecular sieves (200 mg), and 2 mL of dichloromethane were added to the reaction tube, and then dimethyl methylene malonate (R 9 =H, 0.9 mmol) in 2 mL of dichloromethane, reacted at room temperature for 6 hours to obtain product 1b, 135.5 mg, yield 88%, >95 / 5dr. 1 H NMR (400MHz, CDCl 3 )δ7.68(d, J=8.0Hz, 2H), 7.37(d, J=8.0Hz, 2H), 7.16(s, 1H), 6.95(d, J=8.0Hz, 1H), 6.47(d, J=8.4Hz, 1H), 4.23(d, J=1.2Hz, 1H), 3.71–3.65(m, 7H), 3.37(t, J=10.4Hz, 1H), 3.15–3.11(m, 1H), 2.80(s,3H),2.47(s,3H),2.26–2.10(m,5H),1.89–1.84(m,2H),1.79–1.74(m,1H),1.70–1.59(m,1H).
[0116]
Embodiment 3
[0118] Enamine 14c (0.3mmol), indium trichloride (3.3mg), Molecular sieves (100 mg), and 2 mL of dichloromethane were added to the reaction tube, and then dimethyl methylene malonate (R 9 =H, 0.9 mmol) in 2 mL of dichloromethane, reacted at room temperature for 3 hours to obtain product 1c, 110.4 mg, yield 57%, >95 / 5dr. 1 H NMR (400MHz, CDCl 3 )δ7.67(d, J=8.4Hz, 2H), 7.36(d, J=8.4Hz, 2H), 7.29(s, 1H), 7.15(d, J=8.0Hz, 1H), 6.51(d, J=8.0Hz, 1H), 4.65(s, 2H), 4.27(s, 1H), 3.71–3.63(m, 7H), 3.35(t, J=10.4Hz, 1H), 3.13–3.09(m, 1H) ),2.81(s,3H),2.47(s,3H),2.24–2.10(m,2H),1.91–1.82(m,2H),1.79–1.74(m,1H),1.66–1.58(m,1H ),0.95(s,9H),0.12(d,J=2.0Hz,6H).
[0119]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com