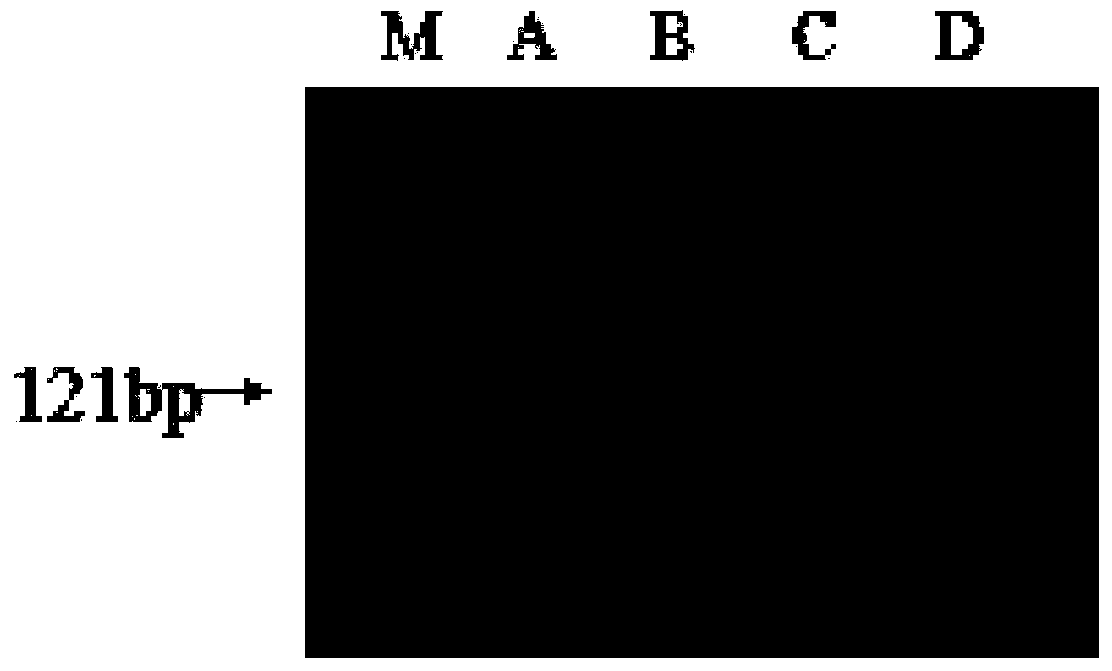Real-time fluorescent quantitative PCR (polymerase chain reaction) detection method for three genotypes of human parvovirus B19, as well as universal detection primer, TaqMan probe and kit thereof
A real-time fluorescence quantitative and detection kit technology, applied in TaqMan probes and kits, real-time fluorescence quantitative PCR detection, and molecular biology detection of nucleic acids, to achieve the effects of programmed operation, convenient sampling, and simple and easy-to-use operation procedures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Design of real-time fluorescent quantitative PCR detection primers and TaqMan probes for simultaneous qualitative and quantitative detection of the three genotypes of human parvovirus B19 (B19 virus)
[0050] According to the gene sequence of the three genotype representative strains of B19 virus in GenBank (GenBank NO.: prototype: M13178; Lali: AY044266; V9: NC-004295), the DNA Man software is used for comparison, and the primers and TaqMan probes are designed In principle, the 121bp NS1 gene conservative region sequence (as shown in SEQ ID NO: 4 in the sequence list) is selected as the detection sequence, and real-time fluorescent quantitative PCR detection primers and TaqMan probes are designed to simultaneously detect the three genotypes of B19 virus to obtain the sequence as follows:
[0051] Upstream primer P (NS1-1): 5'-CGGGACCAGTTCAGGAGAAT-3' (SEQ ID NO: 1 in the sequence table), with a Tm value of 57.4°C;
[0052] Downstream primer P(NS1-2): 5'-CCCAACTAACA...
Embodiment 2
[0057] Example 2. Using the primers of the present invention to perform routine PCR detection on the three genotypes of human parvovirus B19
[0058] 1. Extract the DNA of B19 virus
[0059] The B19 virus international standard plasma (NIBSC code: 09 / 110) containing the three genotypes was used as the test sample (three samples), and the B19 virus was extracted with the High Pure Viral Nucleic Acid Kit (Roche) and referring to the kit instructions DNA.
[0060] 2. Routine PCR detection
[0061] Using the B19 viral nucleic acids of different genotypes extracted in step 1 as templates, PCR amplified 121bp of the conservative region of the NS1 gene under the guidance of the primers P(NS1-1) and P(NS1-2) of the present invention, and 25μL PCR reaction system It is: 10×PCR buffer (formulation: Tris·HCl (pH8.3) 100mM, KCl500mM, MgCl 2 15mM) 2.5μL, dNTPs (2.5mM each) 2μL, 400nM P(NS1-1), 400nM P(NS1-2), DNA template 5μL, 5U Taq enzyme (TaKaRa), add ddH 2 O to 25μL. The PCR reaction conditi...
Embodiment 3
[0062] Example 3. Real-time fluorescent quantitative PCR detection of B19 virus with primers and TaqMan probes of the present invention
[0063] 1. Extraction of B19 virus DNA
[0064] Take two raw plasma and one blood product as the sample to be tested, use the High Pure Viral Nucleic Acid Kit (Roche) and refer to the kit instructions to extract the sample DNA.
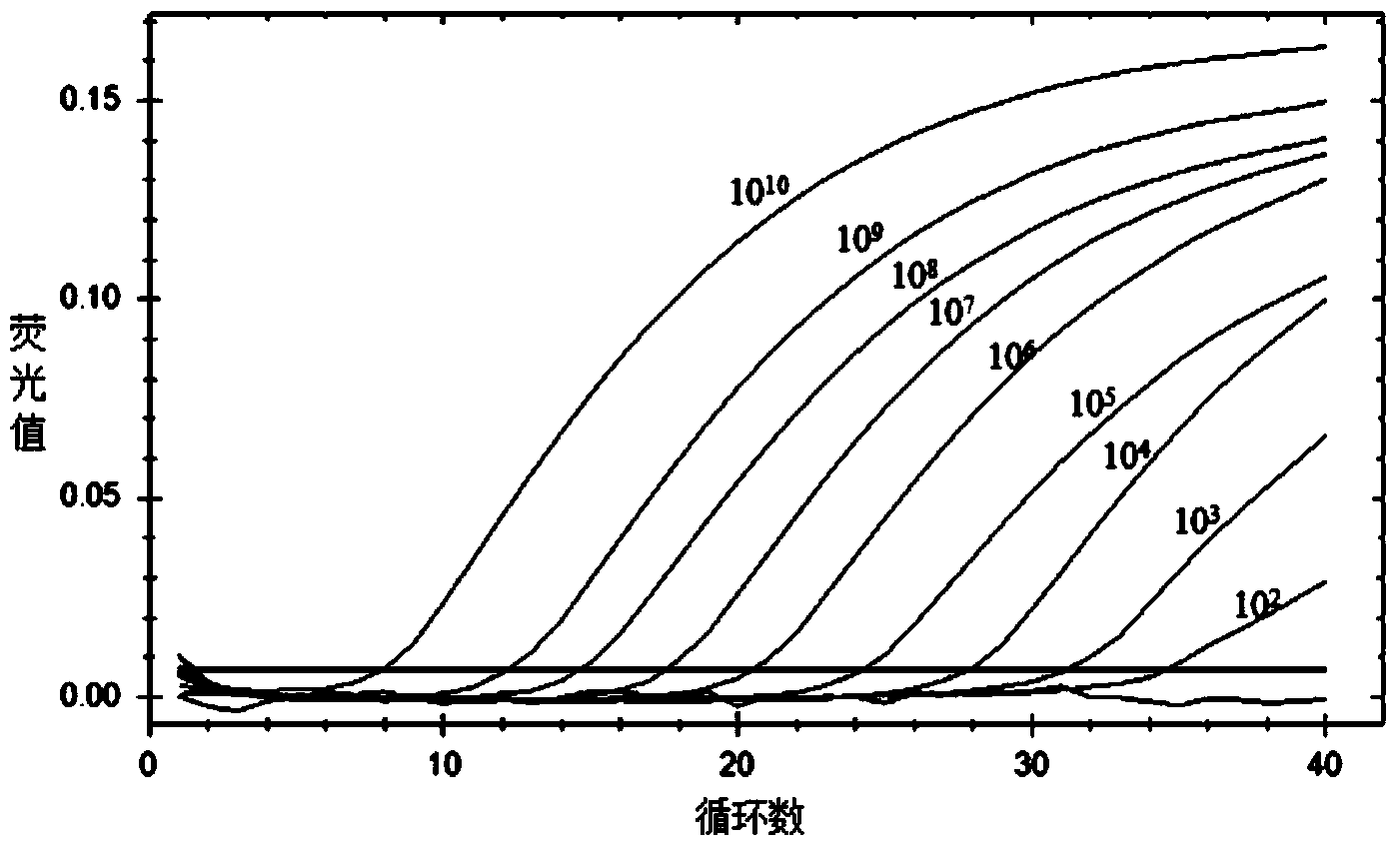
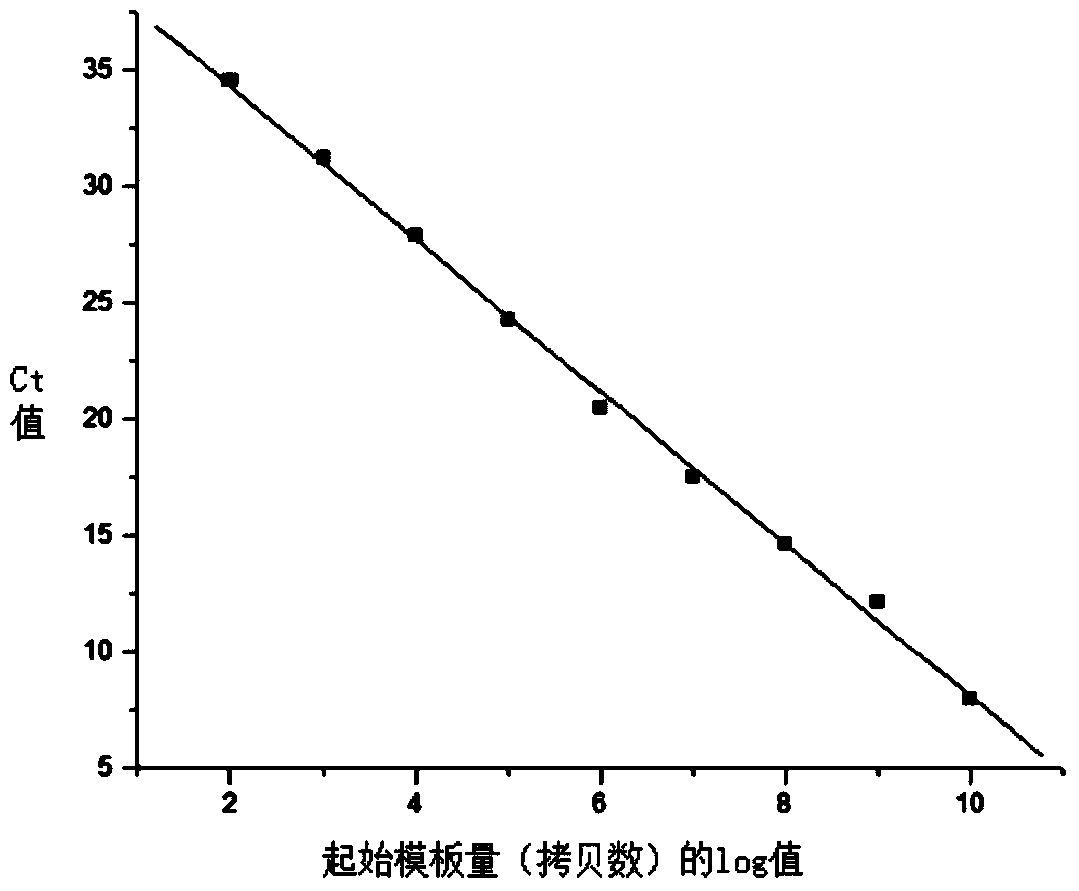
[0065] 2. Preparation of real-time fluorescent quantitative PCR standards
[0066] The target gene fragment for constructing the positive standard quality particle of the present invention is the 121bp B19 virus NS1 gene conserved region, the cloning vector is the pUC19 vector, and the vector size is 2686bp, Amp + For resistance, connect the B19 virus NS1 gene conserved region into the pUC19 vector multi-cloning site between SalI and BamHI restriction sites, and name the recombinant plasmid carrying the B19 virus NS1 gene conserved region B19-pUC19. This sequence was evaluated by Primer Premier5.0 software, and the full B19-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com