Fast joint inspection kit for human immunodeficiency viruses, hepatitis B viruses and hepatitis C viruses and preparation and application thereof
An immunodeficiency virus and hepatitis B virus technology, applied in the field of biotechnology detection, can solve the problems of being unsuitable for clinical testing and large-scale blood screening, time-consuming, and complicated to operate, so as to reduce the risk of cross-contamination and shorten the Experiment period, the effect of high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of kit
[0048] Synthesize HIV detection primers and probes, HBV detection primers and probes, HCV detection primers and probes, and their nucleotide sequences are shown in Table 1 below:
[0049] Table 1
[0050]
[0051] The above primer pairs and probes can be individually packaged, or combined to form a multiplex fluorescent PCR detection mixture. In the multiplex fluorescent PCR detection mixture, the amounts of the above-mentioned primers and probes may be conventional amounts known to those skilled in the art.
[0052] In other words, the kit of the present invention may contain the above-mentioned individually packaged sets of primer pairs and probes, or may contain a configured multiplex fluorescence PCR detection mixture containing each set of primer pairs and probes.
[0053] Further, the kit may also contain dNTP, MgCl 2 , PCR buffer, reverse transcriptase, TaqDNA polymerase, sterile water (DEPC-ddH 2 O), sample genomic DNA / RNA extraction reagen...
Embodiment 2
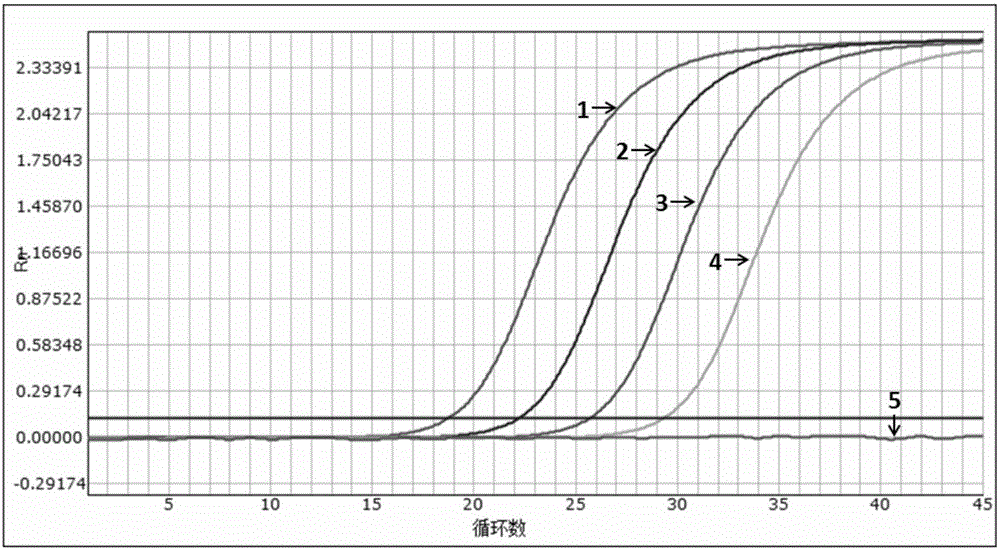
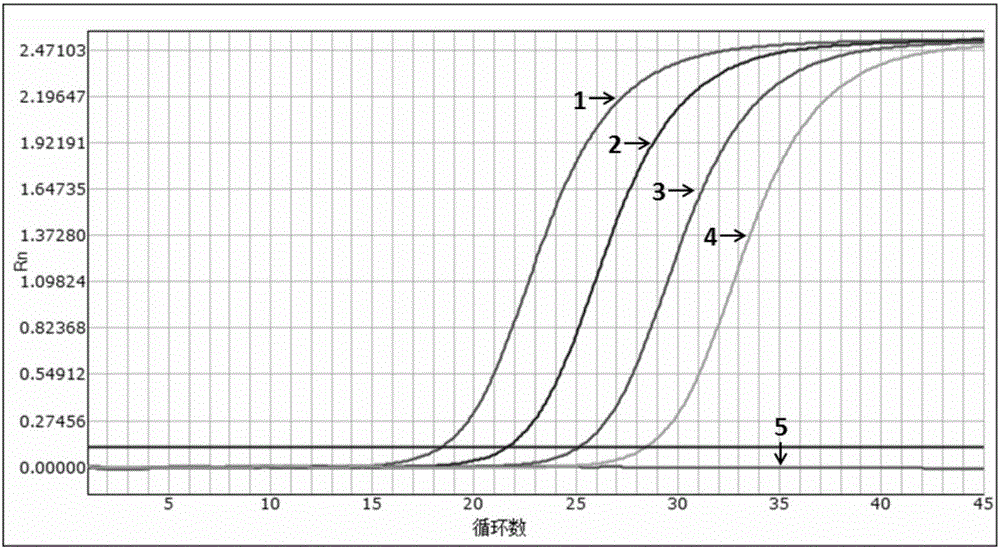
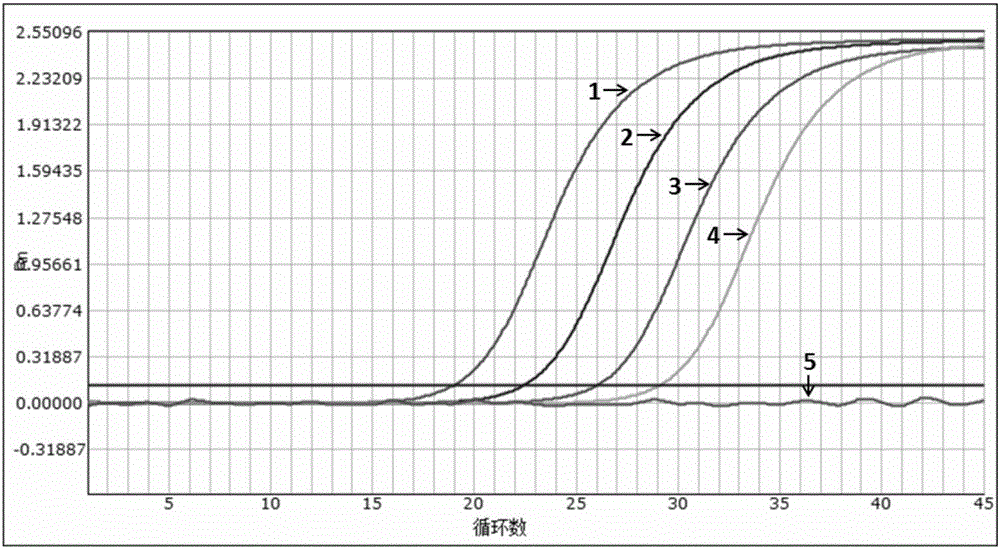
[0054] Example 2 Sensitivity evaluation of the kit
[0055] Experimental purpose: to determine the detection limit (minimum detection concentration) of the multiple fluorescent PCR kit of the present invention (Example 1)
[0056] experimental method:
[0057] 1. Preparation of positive control substance:
[0058] A pseudovirus containing specific amplified fragments of HIV, HBV and HCV was constructed as a positive control. The construction method was carried out with reference to the document "Construction and expression of virus-like particles containing long chimeric RNA in ribonuclease (2008)".
[0059] The nucleic acid sequence of the constructed pseudovirus was verified by Sanger sequencing, and the amplicon fragment containing the HIV, HBV and HCV 3 sets of detection primer pairs in Example 1 can be identified by the aforementioned 3 primers and probes.
[0060] Among them, the nucleotide sequence of the HIV-specific amplified fragment is shown in SEQ ID NO. 10, specifically:
[0...
Embodiment 3
[0086] Example 3 Evaluation of the detection effect of the kit
[0087] experimental method:
[0088] 1. Sample processing:
[0089] The samples used included 2 serum samples from HIV, HBV, and HCV patients, and 2 serum samples from healthy people.
[0090] The above-mentioned serum samples were all extracted using a ribonucleic acid (RNA) extraction kit (magnetic bead column extraction method) produced by Shanghai Zhijiang Biotechnology Co., Ltd.
[0091] The composition of the magnetic bead extraction virus RNA kit is as follows:
[0092] table 5
[0093]
Ingredients
Volume / person
1
Affinity column
1 tube
2
Binding buffer
500μl
3
Washing liquid A
1ml
4
Washing liquid W
1ml
5
Eluent
50μl
6
Magnetic beads
20μl
7
RNA sedimentation aid
6μl
[0094] The specific method of using the magnetic bead method to extract viral RNA kit is as follows:
[0095] ①Prepare reagents: before using the washing solution for the first time, add ethanol according to the instructions on t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com