Extracting method of viral nucleic acid capable of being used for high flux full automation extraction by magnetic bead method
A virus nucleic acid, fully automated technology, applied in the biological field, can solve the problems of lack of versatility, failure to adopt magnetic separation method, and inability to realize high-throughput fully automated operation, so as to achieve simple and fast operation and realize high-throughput automatic operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
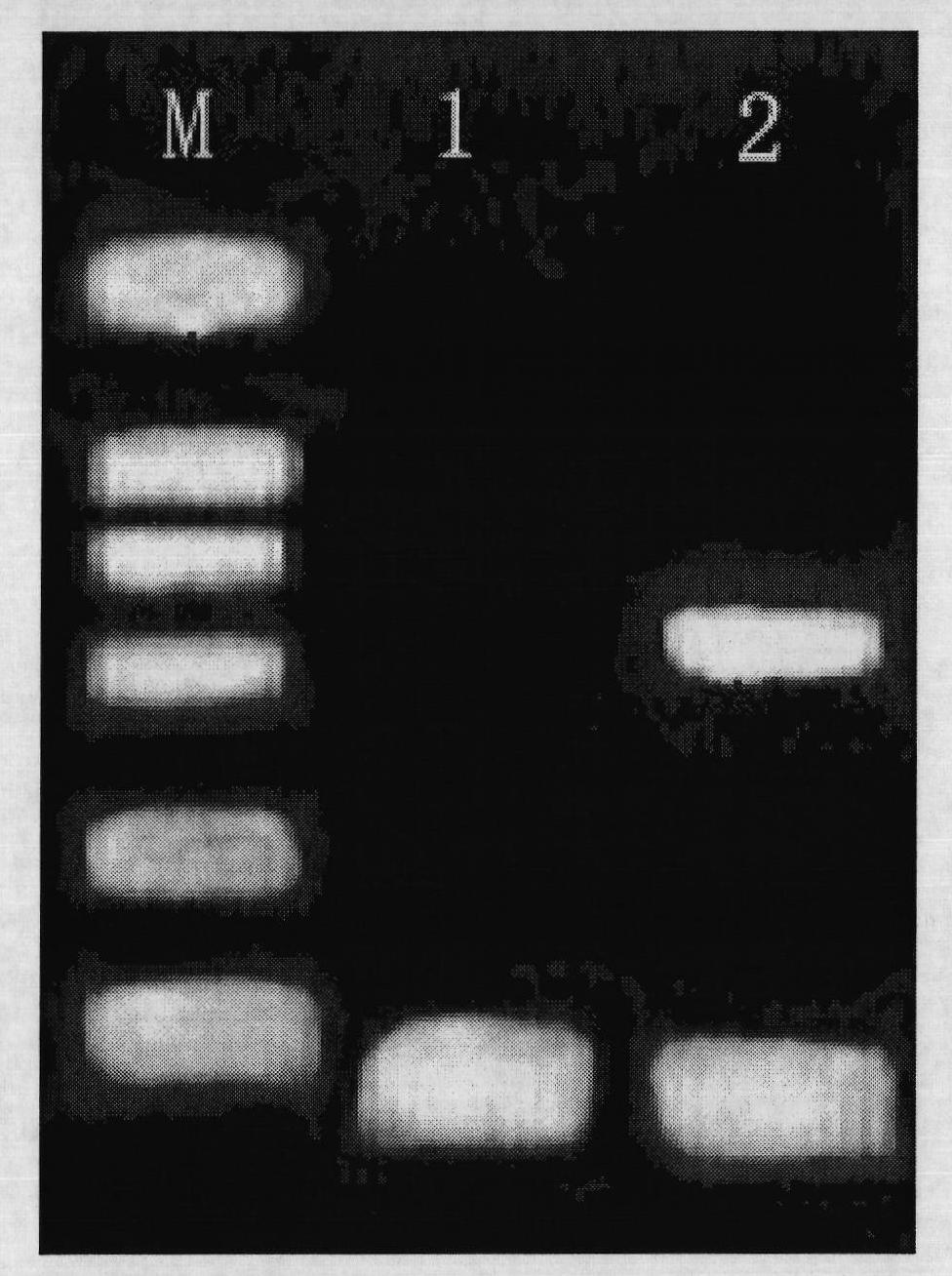
Image
Examples
Embodiment 1
[0032] Step 1: Magnetic Beads I——1.2μm Fe 3 o 4 - Preparation of Hydroxyapatite Magnetic Composite Particles
[0033] Synthesis of 12nm Fe by co-precipitation method reported in literature 3 o 4 magnetic nanoparticles. After mixing 20ml of glacial acetic acid and 780ml of water, add 5g of Fe 3 o 4 Magnetic nanoparticles, ultrasonically dispersed. Under the condition that the stirring speed is 100rpm, add 200ml containing 14.11g Ca(NO 3 ) 2 , 7.02g KH 2 PO 4and 4ml of glacial acetic acid, and stirred for 15 minutes. Slowly add 2mol / L NaOH solution dropwise to the mixed solution to keep the pH of the solution greater than 13. The reaction was carried out for 12 hours under the condition that the stirring speed was 150 rpm. Use a permanent magnet to separate the solid from the reaction solution, and wash the resulting solid 3 times with high-purity water to obtain Fe 3 o 4 - hydroxyapatite magnetic composite particles, the average size of which is 1.2 μm;
[0034] ...
Embodiment 2
[0047] Step 1: Magnetic beads I——Preparation of 0.6 μm Ni-hydroxyapatite magnetic composite particles
[0048] After mixing 25ml of glacial acetic acid and 775ml of water, add 5g of 8nm commercial Ni magnetic nanoparticles and ultrasonically disperse them. Under the condition that the stirring speed is 150rpm, add 200ml containing 8.5g Ca(NO 3 ) 2 , 4.21g KH 2 PO 4 and 7.5ml of glacial acetic acid, and stirred for 30 minutes. Slowly add 2mol / L NaOH solution dropwise to the mixed solution to keep the pH of the solution greater than 13. The reaction was carried out for 6 hours under the condition that the stirring speed was 200 rpm. Use a permanent magnet to separate the solid from the reaction solution, and wash the obtained solid with high-purity water for 3 times to obtain Ni-hydroxyapatite magnetic composite particles with an average size of 0.6 μm;
[0049] Step 2: Extract viral nucleic acid from various biological samples
[0050] The magnetic beads I used in this e...
Embodiment 3
[0055] Step 1: Magnetic Beads I——2μm Ni-Fe 3 o 4 - Preparation of Hydroxyapatite Magnetic Composite Particles
[0056] After getting 40ml of glacial acetic acid and 760ml of water to mix, add 5g of commercialized average size of 8nm Ni magnetic nanoparticles and 5g of commercialized average size of 10nm Fe 3 o 4 Magnetic nanoparticles, ultrasonically dispersed. Under the condition that the stirring speed is 150rpm, add 200ml containing 28.22g Ca(NO 3 ) 2 , 12.64g KH 2 PO 4 and 8ml of glacial acetic acid, stirred for 30 minutes. Slowly add 4mol / L NaOH solution dropwise to the mixed solution to keep the pH value of the solution greater than 13. The reaction was carried out for 6 hours under the condition that the stirring speed was 200 rpm. Use a permanent magnet to separate the solid from the reaction solution, and wash the resulting solid with high-purity water for 3 times to obtain Ni-Fe 3 o 4 - hydroxyapatite magnetic composite particles, the average size of which...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com