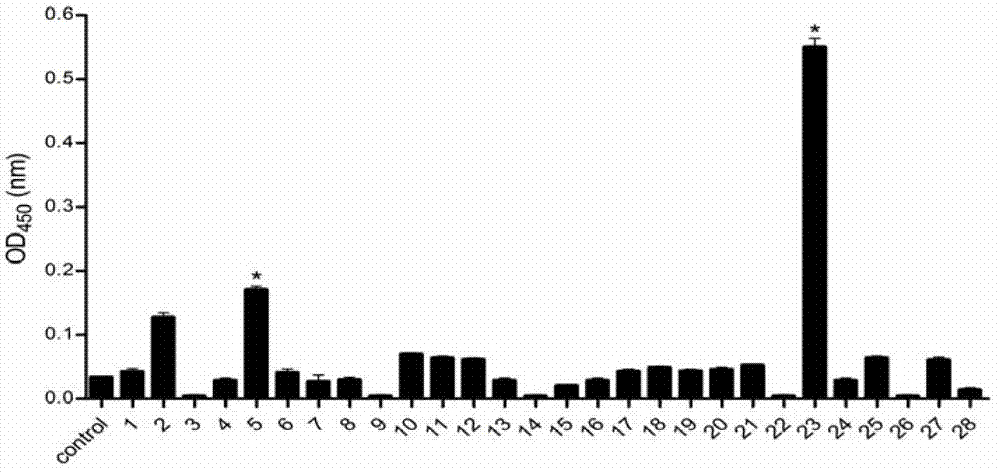
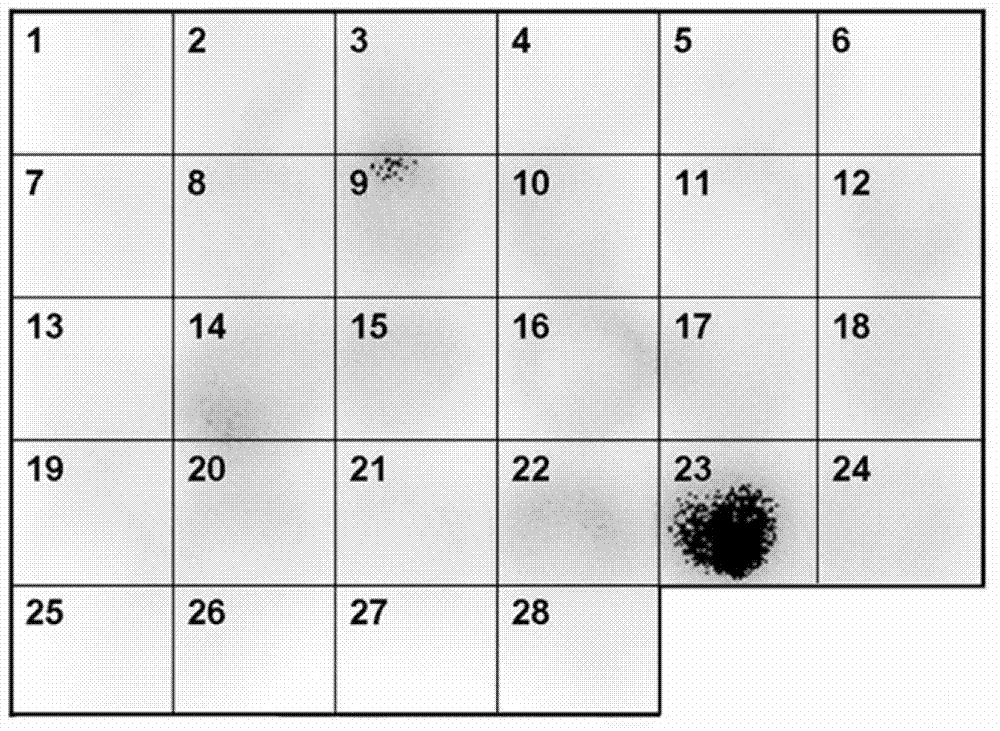
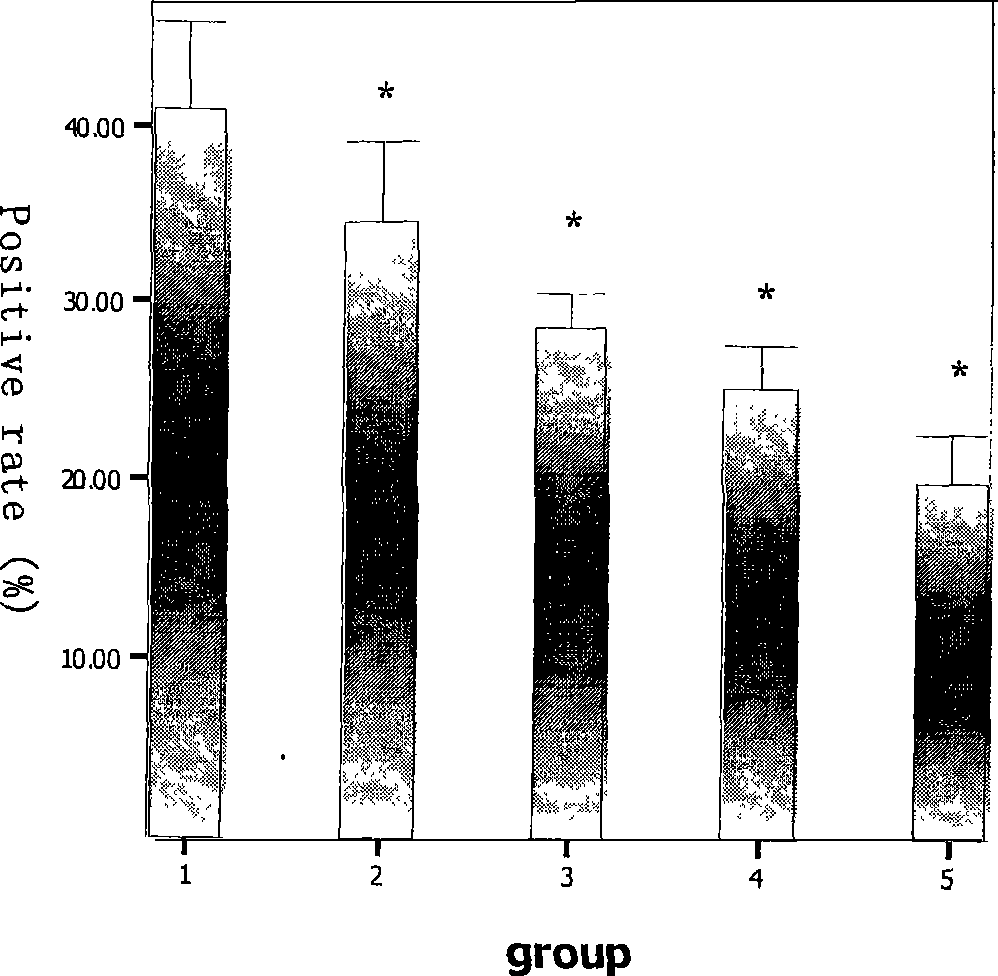
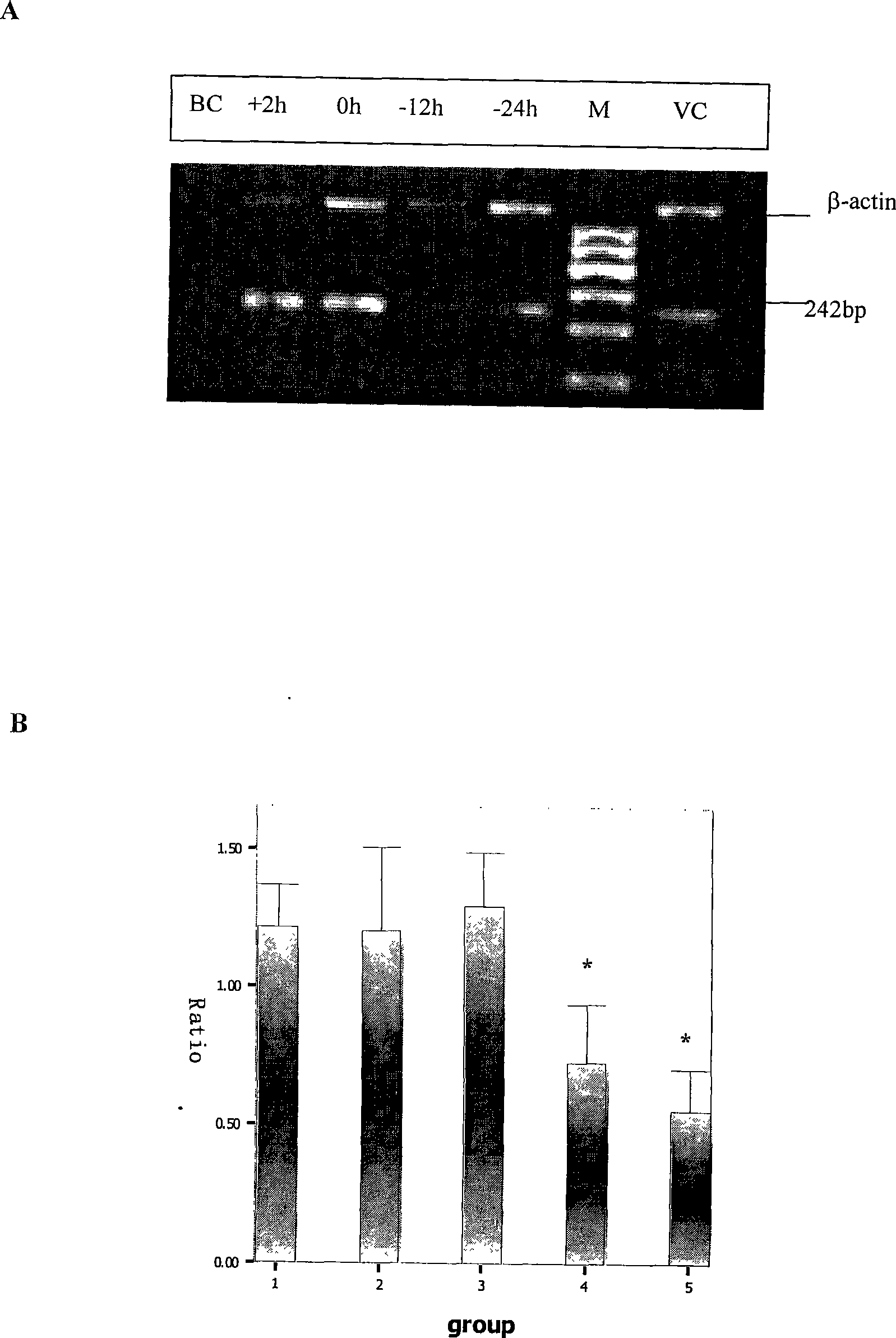
Patents
Literature
119 results about "Hemorrhagic Fevers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
<p>Viral hemorrhagic fevers (VHFs) are a group of illnesses caused by four families of viruses. These include the <a href='https://medlineplus.gov/ebola.html'>Ebola</a> and Marburg, Lassa fever, and yellow fever viruses. VHFs have common features: they affect many organs, they damage the blood vessels, and they affect the body's ability to regulate itself. Some VHFs cause mild disease, but some, like Ebola or Marburg, cause severe disease and death.</p> <p>VHFs are found around the world. Specific diseases are usually limited to areas where the animals that carry them live. For example, Lassa fever is limited to rural areas of West Africa where rats and mice carry the virus.</p> <p>The risk for travelers is low, but you should avoid visiting areas where there are disease outbreaks. Because there are no effective treatments for some of these viral infections, there is concern about their use in <a href='https://medlineplus.gov/biodefenseandbioterrorism.html'>bioterrorism</a>.</p> <p >Centers for Disease Control and Prevention</p>
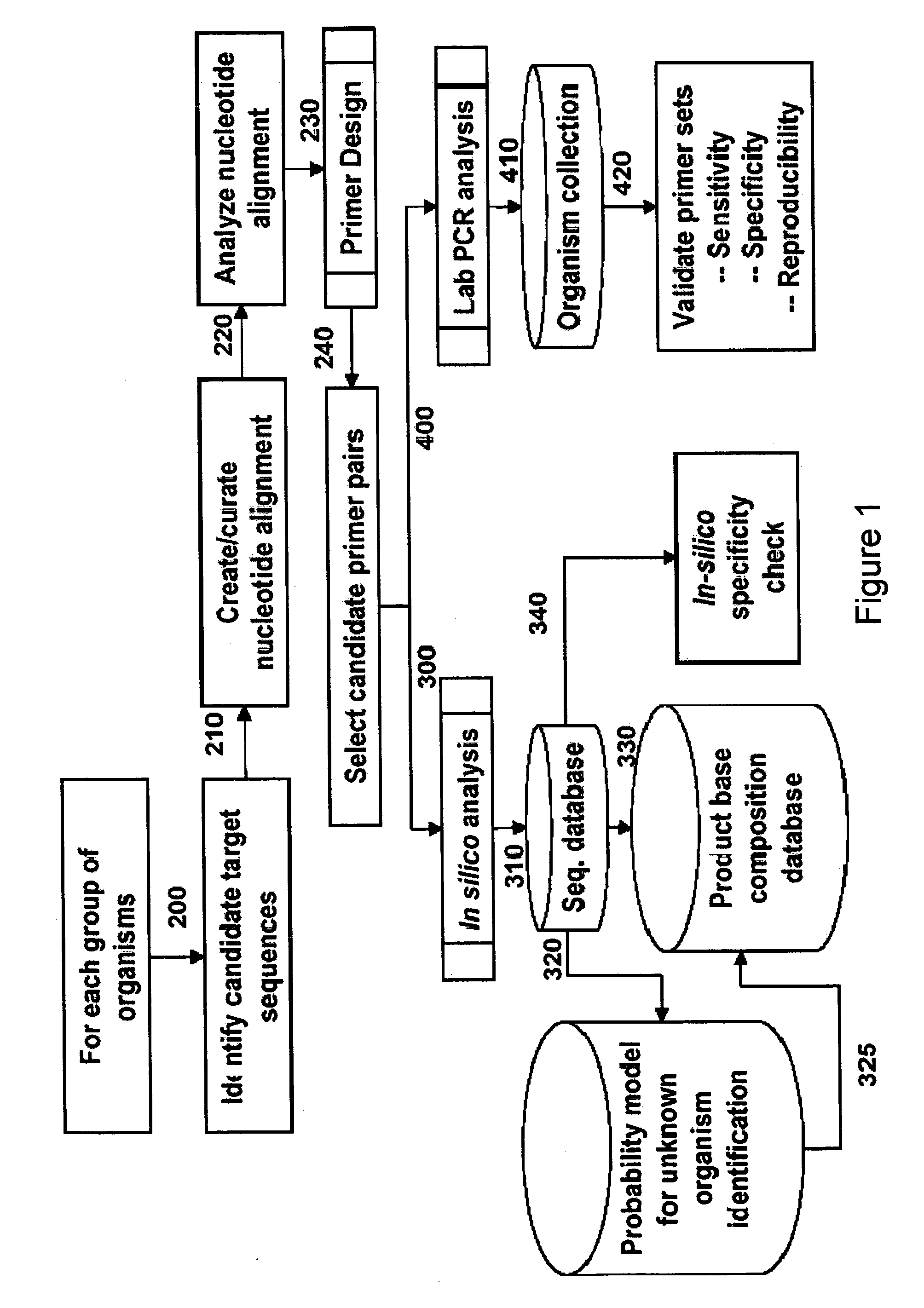
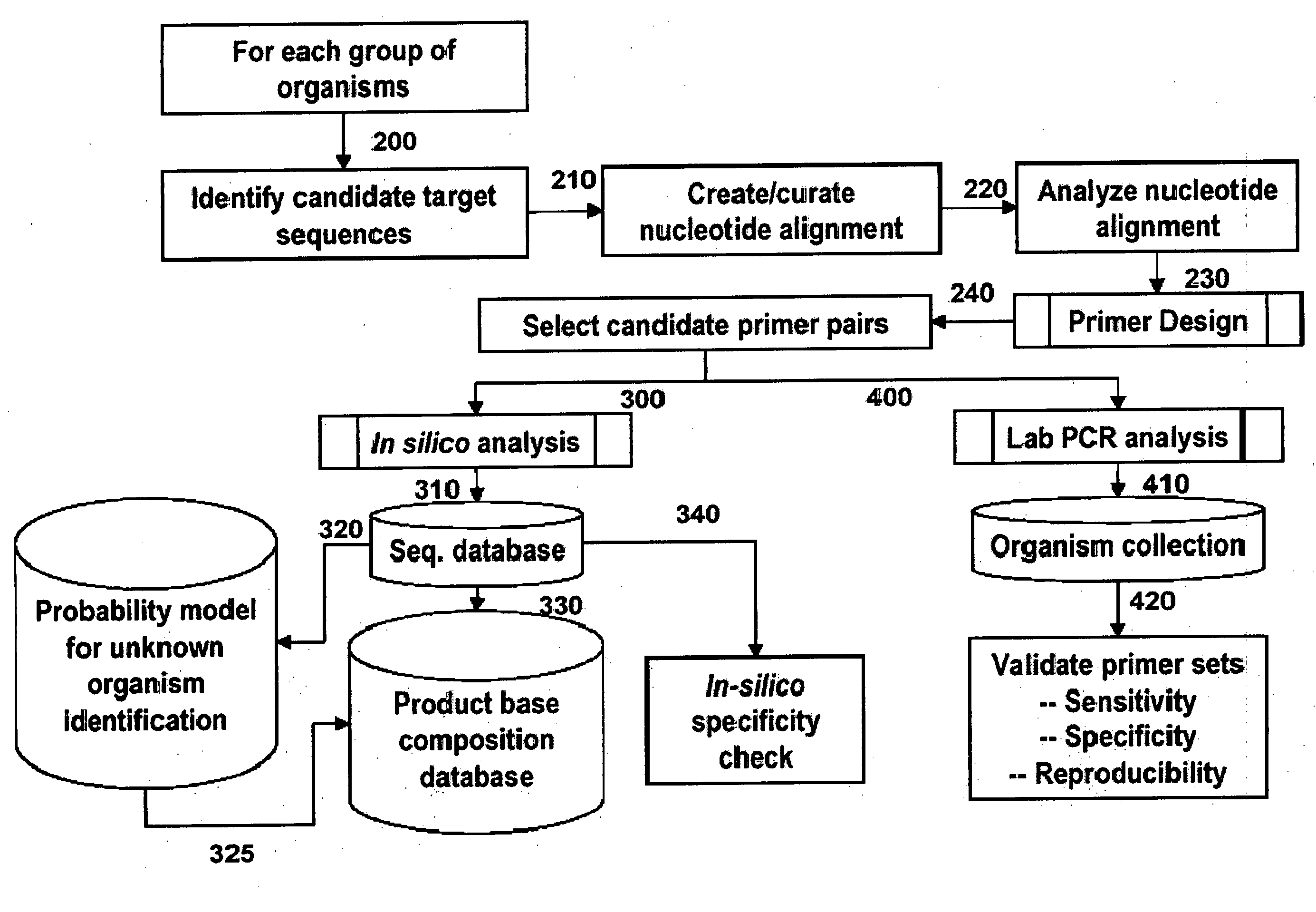
Compositions for use in identification of viral hemorrhagic fever viruses
InactiveUS7312036B2Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses that cause viral hemorrhagic fevers by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Compositions for use in identification of viral hemorrhagic fever viruses
InactiveUS20060057605A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses that cause viral hemorrhagic fevers by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
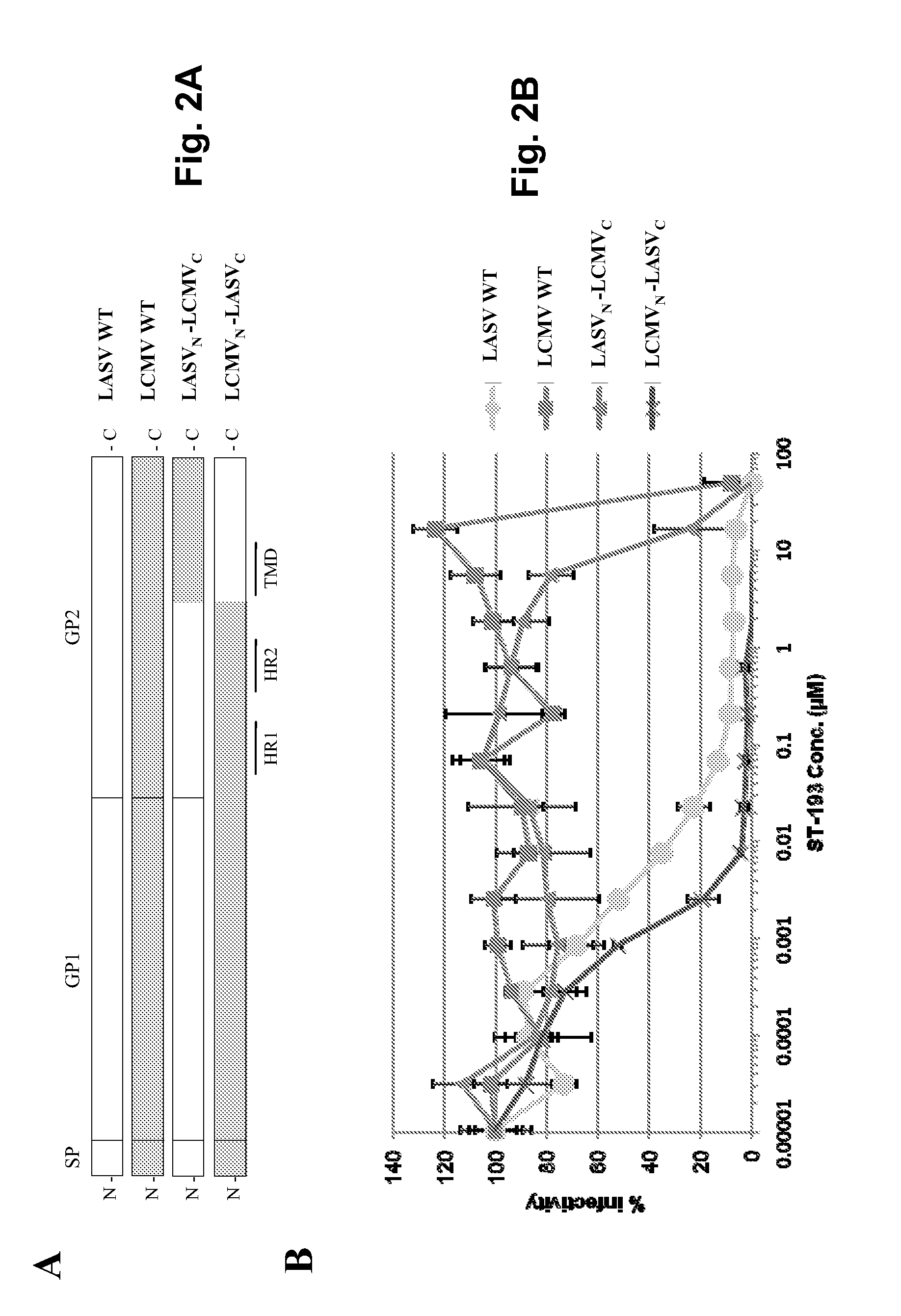
Antiviral drugs for treatment of arenavrus infection
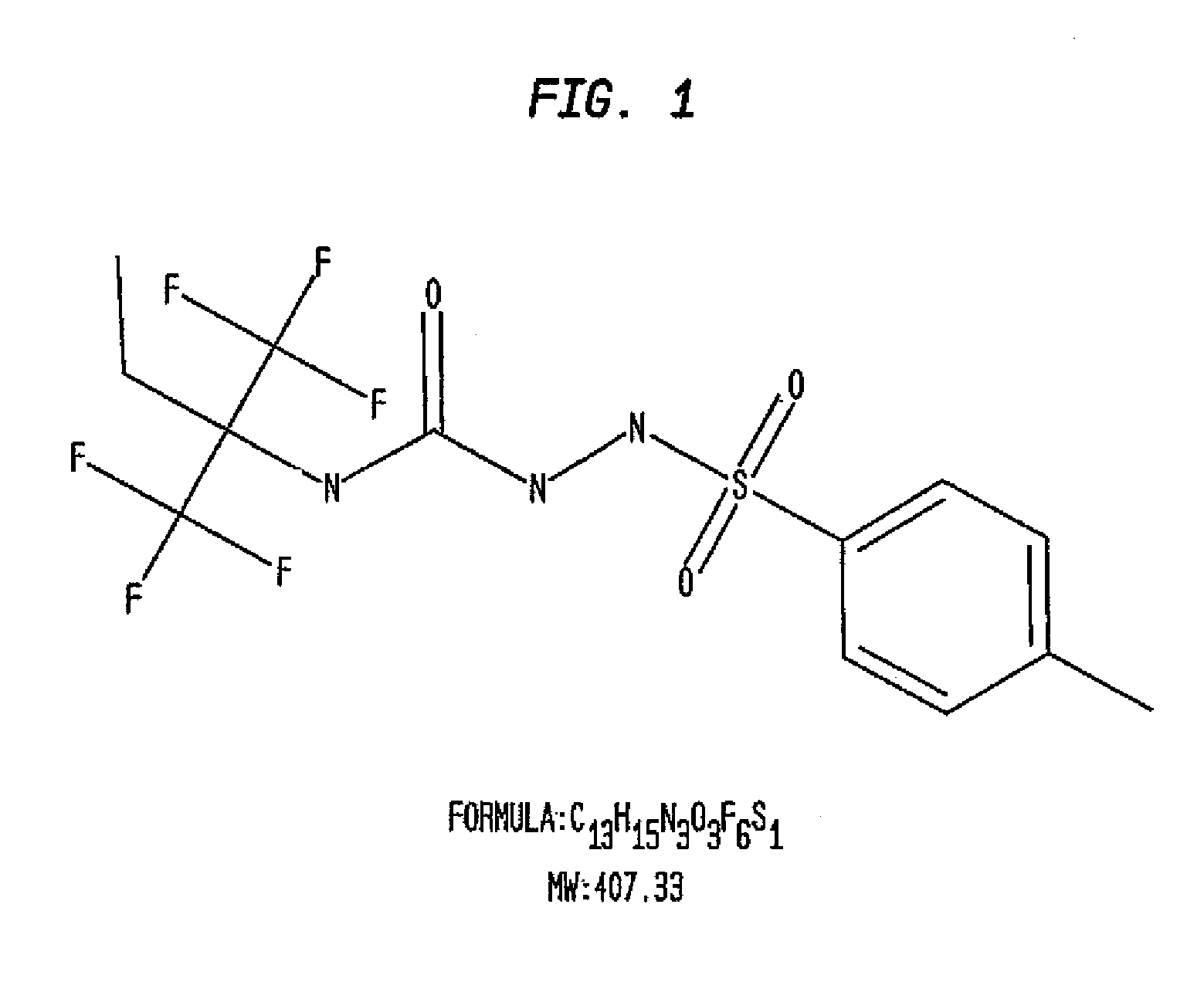
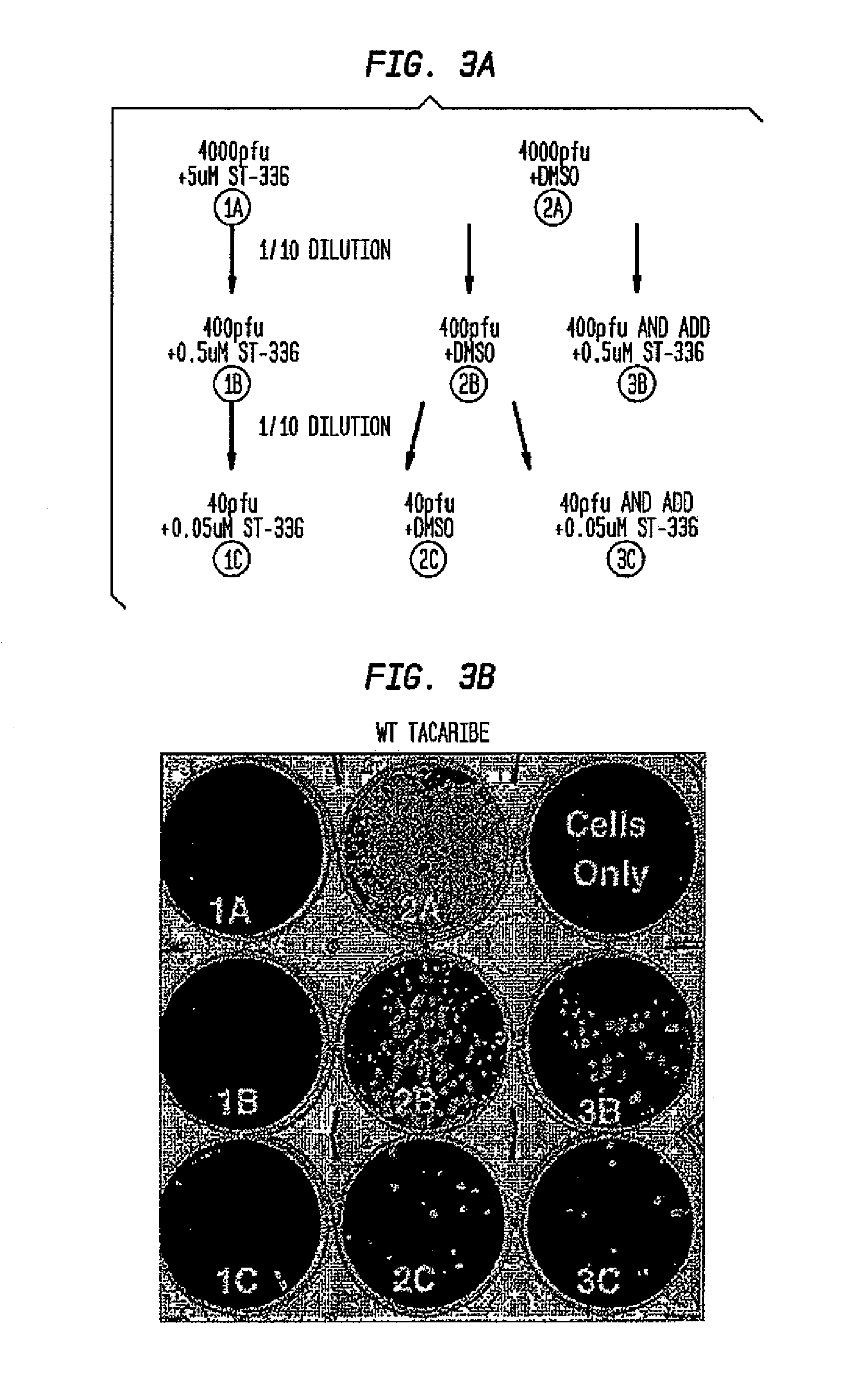
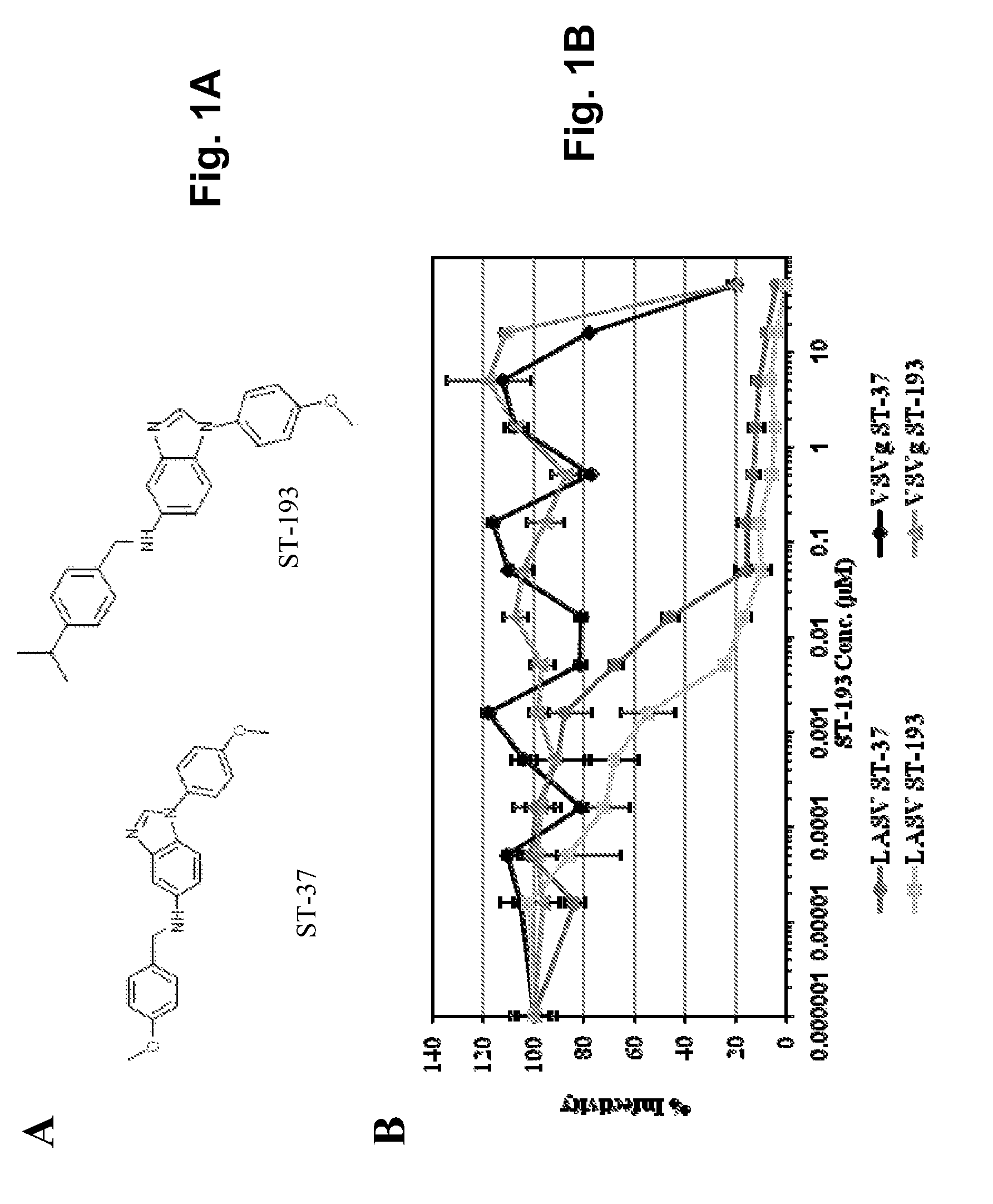
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain novel compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by hemorrhagic fever viruses is disclosed, i.e., including but not limited to, Arenaviridae (Junin, Machupo, Guanarito, Sabia, Lassa, Tacaribe, Pichinde, and LCMV), Filoviridae (Ebola and Marburg viruses), Flaviviridae (yellow fever, Omsk hemorrhagic fever and Kyasanur Forest disease viruses), and Bunyaviridae (Rift Valley fever and Crimean-Congo hemorrhagic fever).
Owner:KINETA FOUR LLC
Compositions and methods for treatment of filovirus-mediated diseases
The invention features compositions, methods, and kits useful for the treatment of filovirus-mediated diseases, e.g., hemorrhagic fever caused by Ebola virus, in an animal.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Agents for the inhibition of virus replication through regulation of protein folding
The invention concerns agents for the treatment of acute and chronic infections with human and animal pathogenic viruses which assemble along the cell membrane and are released through budding on the surface of the cell. Hereunto count especially causative agents of infectious diseases such as AIDS, hepatitis, hemorrhagic fever, SARS, smallpox, measles, polio or the flu. The subjects of the invention are agents that contain inhibitors of the protein folding as active components. Hereunto count inhibitors of cellular folding enzymes (the enzymatic chaperones) as well as substances that disturb the folding of proteins through chemical chaperones. The following substance classes and their derivates belong thereunto: Geldanamycin, Deoxyspergualin, 4-PBA or Herbimycin A. Due to these agents the highly organised processes of the assembly and the proteolytical maturation of virus structure proteins is disturbed. As a result the release and production of infectious decendent viruses is prevented.
Owner:VIROLOGIK GMBH
Cultivation method for nano-selenium-containing iron goddess tea organic tea tree and nano-selenium-containing iron goddess tea organic tea
The invention aims to provide a cultivation method for nano-selenium containing Tie Guanying organic tea plant and the nano-selenium containing Tie Guanying organic tea plant. One hundred grams of organic tea leaves obtained through the cultivation method contains 40 to 65 micrograms of nano-selenium with a granular diameter of 50 to 200 nanometers, 4.36 to 5.45 grams of ether extracts and the balance dietary fibers, carbohydrates, proteins, microelements such asmanganese, magnesium, phosphorus, zinc, iron, copper, sodium, calcium and potassium, and vitamins including A, A1, B1, B2, B5, E and renieratene. The organic tea leaves meet standards of Green Organic Tea Certification and requirements and standards of organic tea production, processing and trade. Selenium has functions of free radical removal, prevention of cancers, beauty treatment and aging resistance, weight reduction, radiation resistance, adjustment of immunity, treatment of Keshan disease, etc., and nutritional anticancer agent accepted by medical community. The organic tea leaves, after long term drinking, have better prevention and cure effect on hepatitis, arthritis, cataract, angiocardiopathy and hemorrhagic fever and other diseases and is benefit to health and longevity of the middle-aged and old.
Owner:张金松
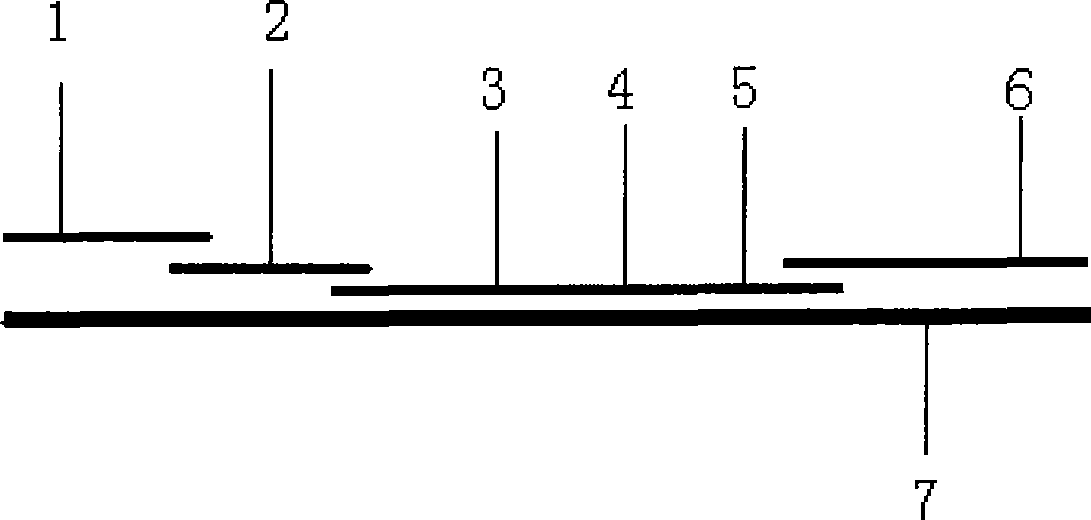
Kidney syndrome blooding diagnosis test paper strip, preparing method and detection reagent kit thereof
The invention provides a diagnostic strip for hemorrhagic fever with renal syndrome, and a preparation method and a detection reagent kit thereof. The diagnostic strip is a colloidal gold immunochromatographic strip which is formed by that a sample absorption pad (1), a colloidal gold bonding pad (2), a pyroxylin film (5) and a water absorption pad (6) are sequentially stuck on a PVC soleplate (7) in a mutual lapping way. The diagnostic strip is characterized in that the colloidal gold bonding pad (2) is composed of glass-fiber membranes of goat anti-human Mu-chain immune gold complexes containing colloidal gold markers, a detection area (3) and a quality control area (4) are arranged on the pyroxylin film (5), the position of the detection area (3) is coated with hantavirus nucleocapsid protein antigens, and the position of the control area (4) is coated with rabbit anti-goat IgG antibodies. The diagnostic strip has good specificity, sensitivity and repeatability, can realize the early and rapid diagnosis of HFRS, and is easy to carry; and the operation is simple, the cost is low, and the response is fast. The invention is easy to be popularized in primary medical units.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system
ActiveCN102191338AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceYellow fever
The invention discloses a fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system consisting of primers, probes, a Premix Ex Taq reaction solution and a sterilized Tris buffer. With good singularity and high sensitivity, three pairs of primers and probes are very suitable for simultaneously detecting viruses of yellow fever, dengue fever and epidemicencephalitis B. And there is no cross reaction between the primers and probes and several other entomophily hemorrhagic fever viruses, such as Marburg virus and Rift Valley fever virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Compositions and methods for treatment of filovirus-mediated diseases
InactiveUS8475804B2Slow and stop replicationReduce loadBiocideOrganic chemistryEbola virusTreated animal
The invention features compositions, methods, and kits useful for the treatment of filovirus-mediated diseases, e.g., hemorrhagic fever caused by Ebola virus, in an animal.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Multiplex fluorescent polymerase chain reaction (PCR) kit and primers for detecting Ebola viruses, Marburg viruses, Lassa viruses and Rift Valley fever viruses
InactiveCN102719557AAccurate detectionControl incomingMicrobiological testing/measurementFluorescence/phosphorescenceMarburg virusRift Valley fever virus
The invention provides a multiplex fluorescent polymerase chain reaction (PCR) kit and primers for detecting Ebola viruses, Marburg viruses, Lassa viruses and Rift Valley fever viruses. The multiplex fluorescent PCR kit comprises conventional reagents of an RT-PCR buffer and an RT-PCR enzyme mixed liquor and also comprises primers and probes for detecting the four viruses, wherein the primers are shown in sequences of SEG ID NO: 1-13 and the probes are shown in sequences of SEQ ID NO: 14-18. The multiplex fluorescent PCR kit, the primers and the probes realize rapid and accurate detection of pathogens of Ebola hemorrhagic fever, Marburg hemorrhagic fever, Lassa fever and Rift Valley fever, prevent the four infectious diseases from spreading into or out of the frontier port, are accurate and effective, have strong operability, and can be used for detection of the infectious diseases. Through the multiplex fluorescent PCR kit, the primers and the probes, suspect cases can be found timely and a capability of preventing the infectious diseases from spreading into our country is improved.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
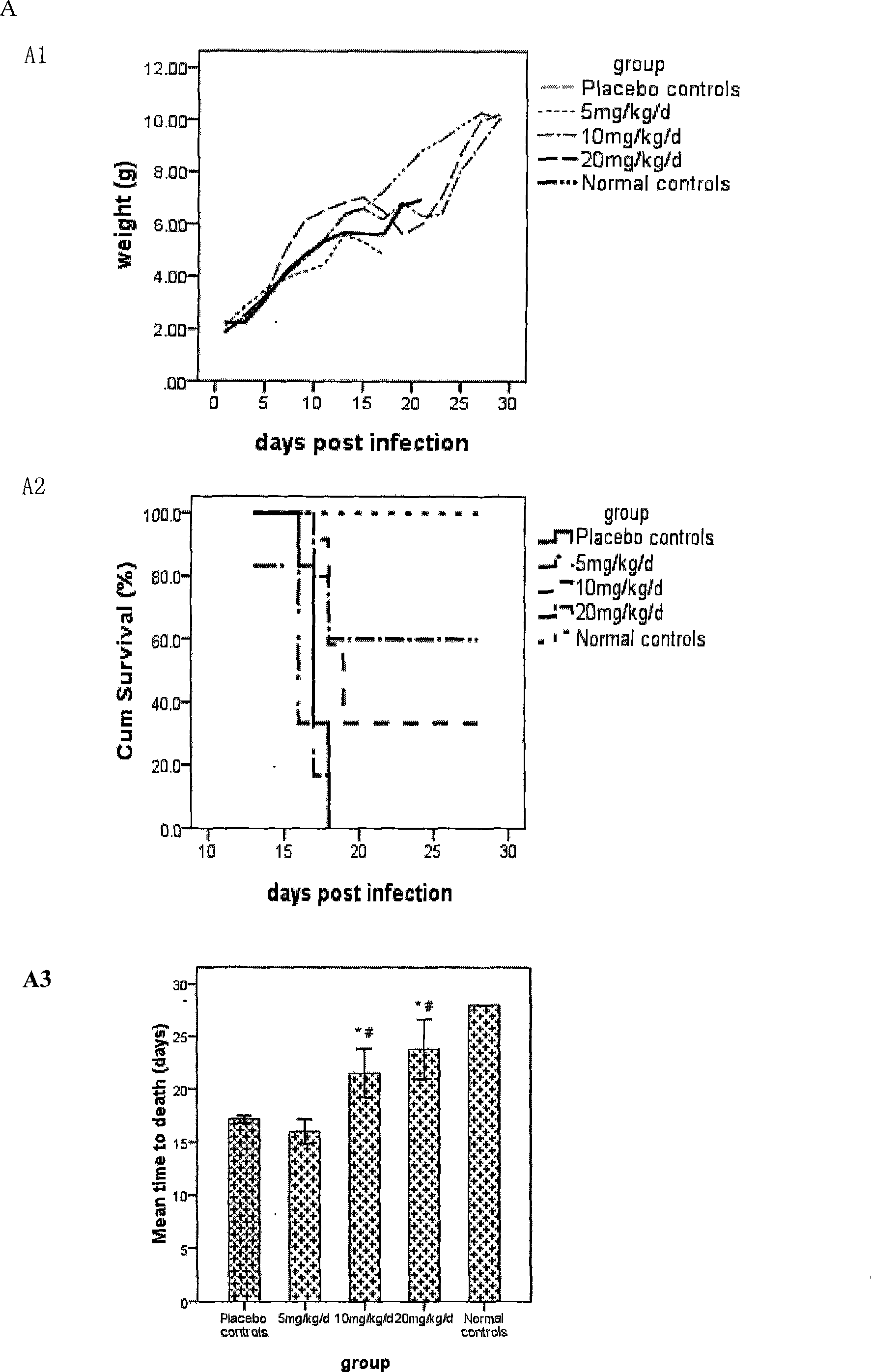
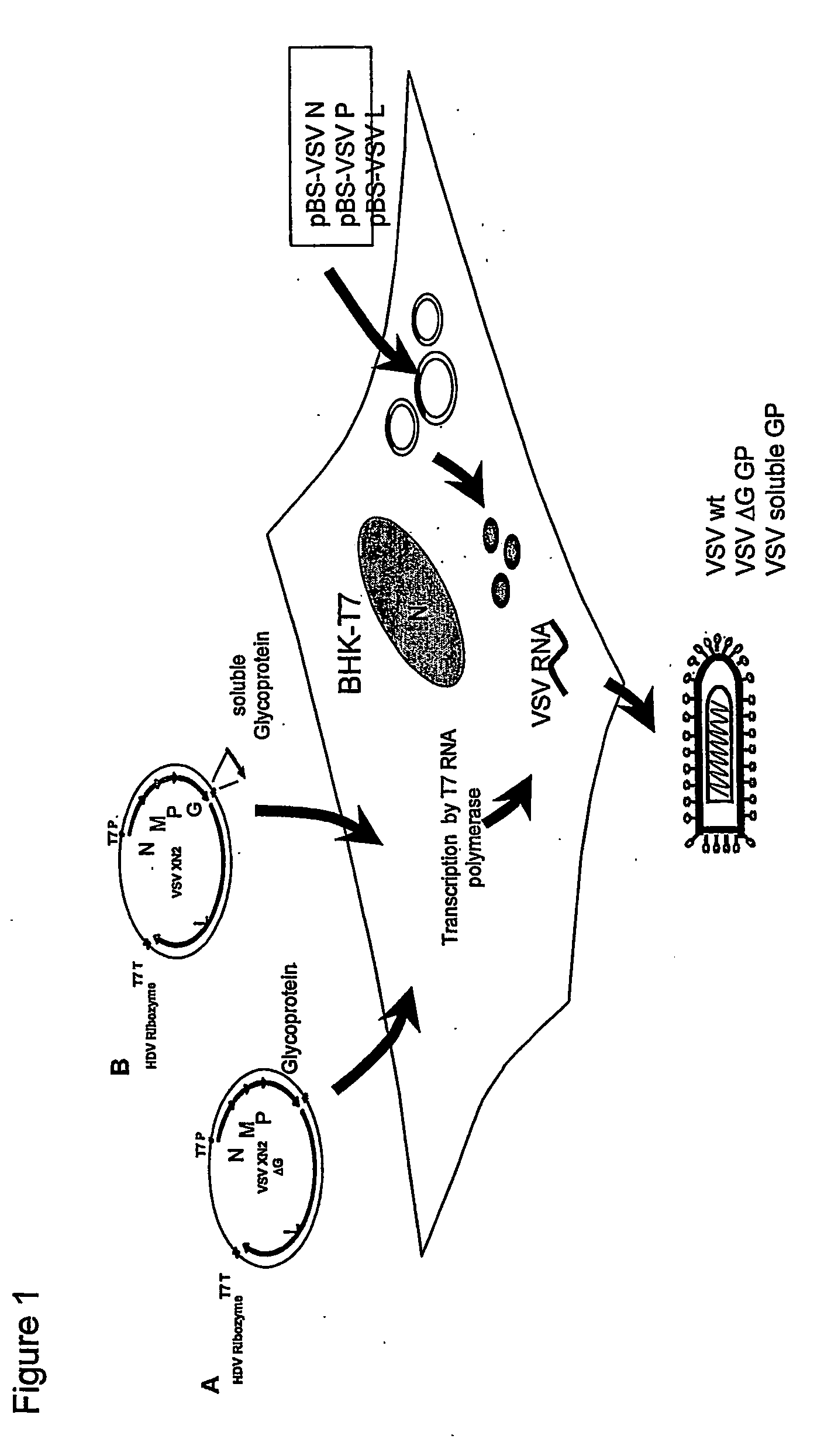
Recombinant vesicular stomatitis virus vaccines for viral hemorrhagic fevers
InactiveUS8012489B2SsRNA viruses negative-senseViral antigen ingredientsViral VaccineVesicular stomatitis virus
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Antihepatitis medicament, preparation method thereof and use thereof
ActiveCN101633683AEasy to slidePromote dissolutionOrganic active ingredientsDigestive systemSolubilityAllergic purpura
The invention relates to a glycyrrhizic acid derivative, preparation thereof and use thereof. The derivative has high water solubility and high storage stability and is suitable to be used in medicaments and health-care products for treating or preventing hepatitis, liver cirrhosis, hemorrhagic fever of liver damage and liver dysfunction syndrome, allergic purpura, psoriasis vulgaris, eczematousdermatitis and the like.
Owner:刘力
Marburg and Ebola dual-virus fluorescent quantitative PCR (Polymerase Chain Reaction) detection method and system
ActiveCN102140533AMicrobiological testing/measurementFluorescence/phosphorescenceEbola virusYellow fever
The invention discloses Marburg and Ebola dual-virus fluorescent quantitative PCR (Polymerase Chain Reaction) detection method and system, wherein the detection system comprises primers, probes, a Premix EX Taq reaction solution and sterilizing Tris water. As two pairs of primers and probes have very good specificity, the detection system has high sensitivity and is suitable for simultaneously detecting Marburg and Ebola viruses without having cross reaction with other kinds of hemorrhagic fever arbovirus, such as yellow fever, dengue and rift valley fever.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Compositions and methods for treatment of filovirus-mediated diseases
InactiveUS20130289024A1Slow and stop replicationReduce loadAntiviralsHeterocyclic compound active ingredientsEbola virusPediatrics
The invention features compositions, methods, and kits useful for the treatment of filovirus-mediated diseases, e.g., hemorrhagic fever caused by Ebola virus, in an animal.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
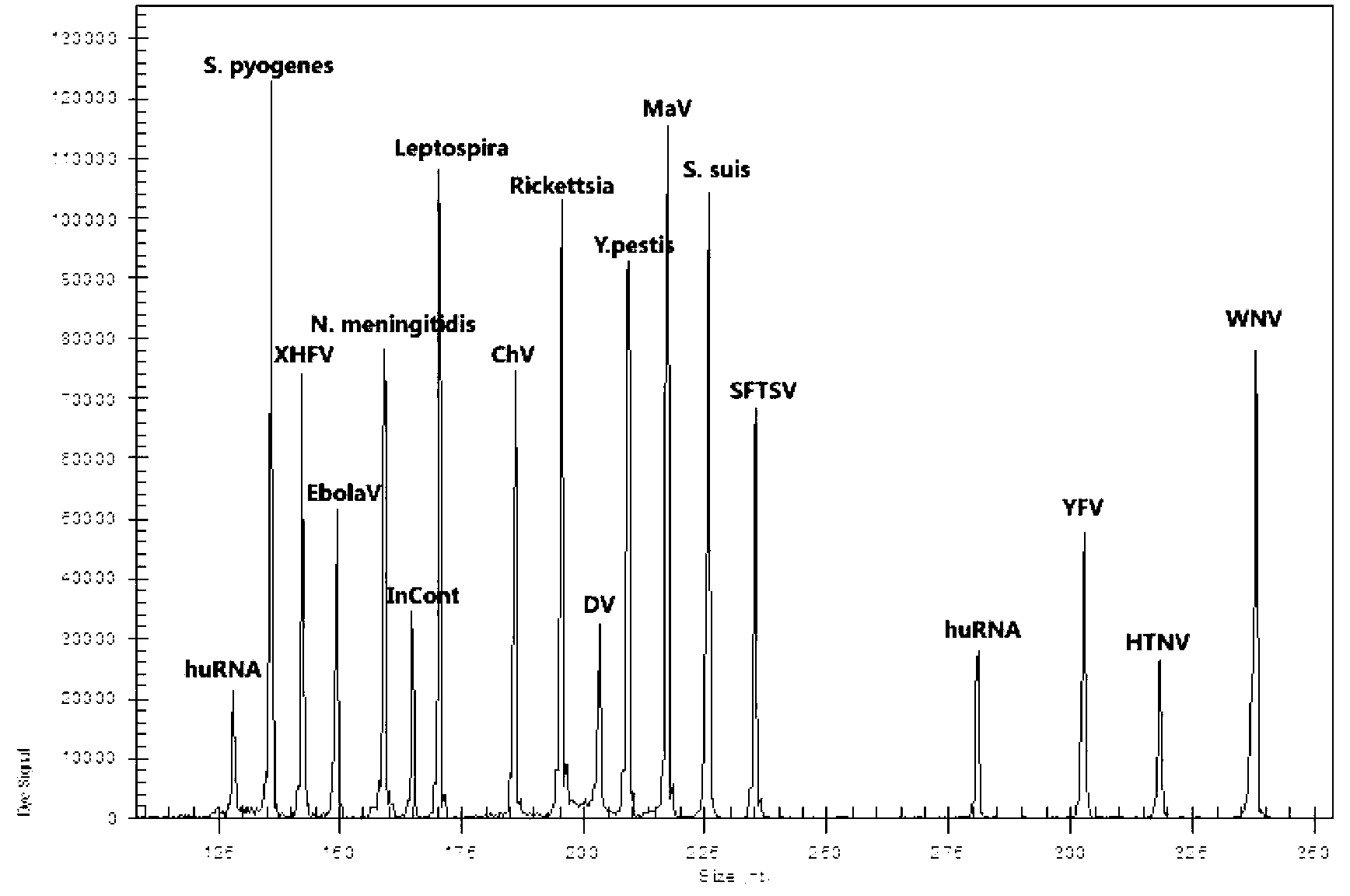
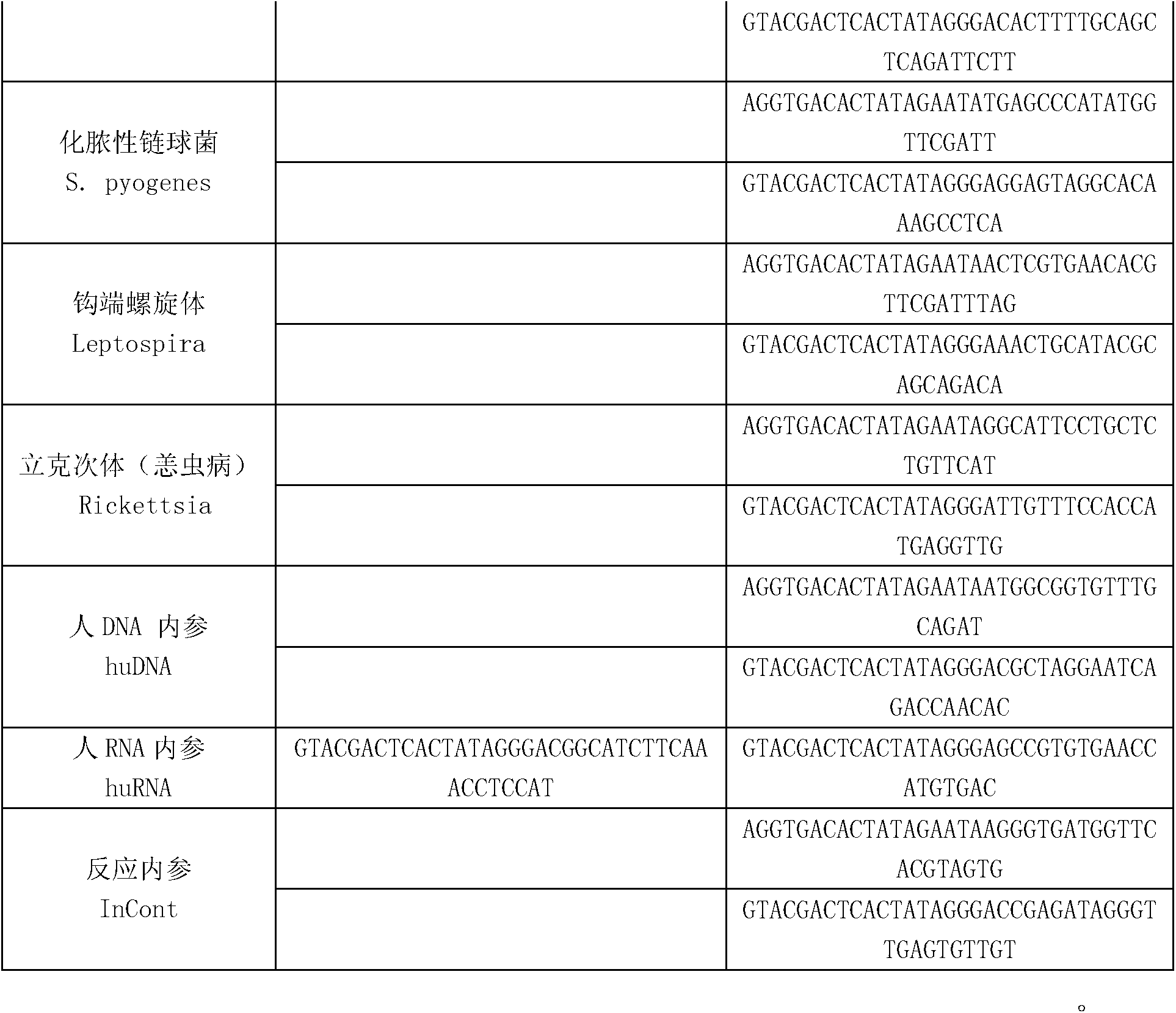
Kit for synchronously detecting fifteen hemorrhagic fever pathogens and detection method of kit
ActiveCN103074452AMonitor reaction efficiencyEnsuring Quality JudgmentsMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting fifteen hemorrhagic fever pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine hemorrhagic fever pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-10 (sequence identifier number 1-10), and the PCR primer comprises forward and reverse PCR amplification primers of the rest six hemorrhagic fever pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the nine hemorrhagic fever pathogens and the human RNA internal reference, and has a gene sequence show as SEQ ID NO. 10-36. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
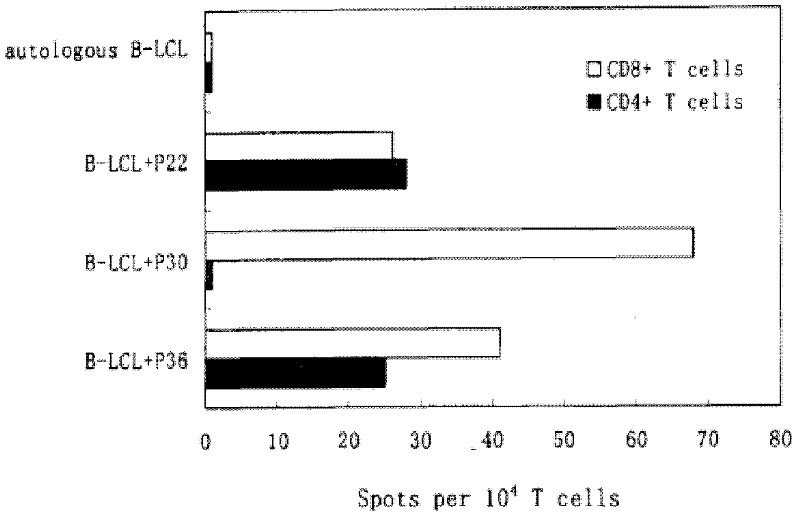
HTNV-NP (Hantaan virus nucleoprotein)-specific CTL (cytotoxic T lymphocyte) epitope peptides and application thereof
The invention discloses HTNV-NP (Hantaan virus nucleoprotein)-specific CTL (cytotoxic T lymphocyte) epitope peptides and application thereof. The CTL epitope peptides have amino acid sequences shown in SEQ ID NO:1-12. Especially, HLA-I (human leucocyte antigen-I) molecule restricted epitope polypeptide in the HTNV-NP-specific CTL epitope peptides can induce CD8+T lymphocyte to generate strong cellular immune response and secrete high-level IFN-gamma. The HTNV-NP-specific CTL epitope peptides can be used for preparing CTL epitope peptide vaccines or for inducing the generation of CTL epitope peptide-specific CTL, or for preparing CTL epitope peptide-sensitized antigen presenting cells and have bright development and application prospects in the field of specific immunization therapy of HFRS (hemorrhagic fever with renal syndrome).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
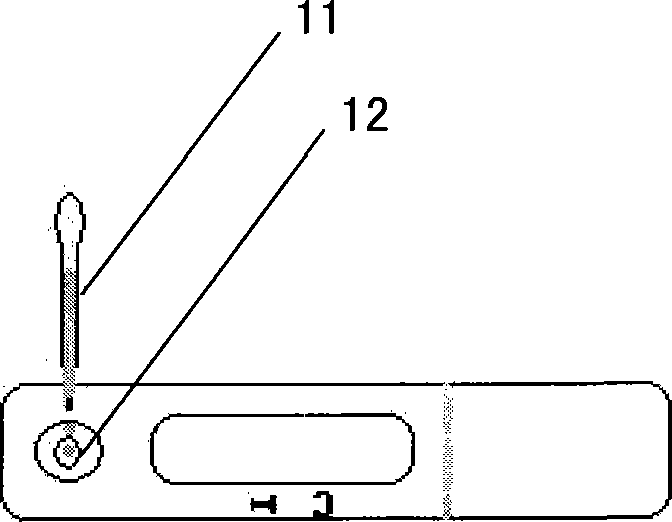
Detection method of hemorrhagic fever with renal syndrome IgM antibodies and reagent kit
ActiveCN103033616AReduce the impact of stabilityExtended shelf lifeMaterial analysisSerum igeIgm antibody
The invention relates to the technical field of biological detection, in particular to a detection method of hemorrhagic fever with renal syndrome IgM (immunoglobulin M) antibodies and a reagent kit. The detection method comprises the steps that hemorrhagic fever with renal syndrome antigens are fixed on a membrane; to-be-detected serum is added; the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum are combined with the antigens fixed on the membrane; gold labeled operating fluid containing mouse anti-human IgM monoclonal antibodies is added; the mouse anti-human IgM monoclonal antibodies are combinec with the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum; a red color is shown; scrubbing liquid is added finally; and the redundant gold labeled operating fluid and other impurities are washed out. The reagent kit has the characteristics of longer quality guarantee period, less cross reaction, better color rendering performance and the like.
Owner:山东康华生物医疗科技股份有限公司
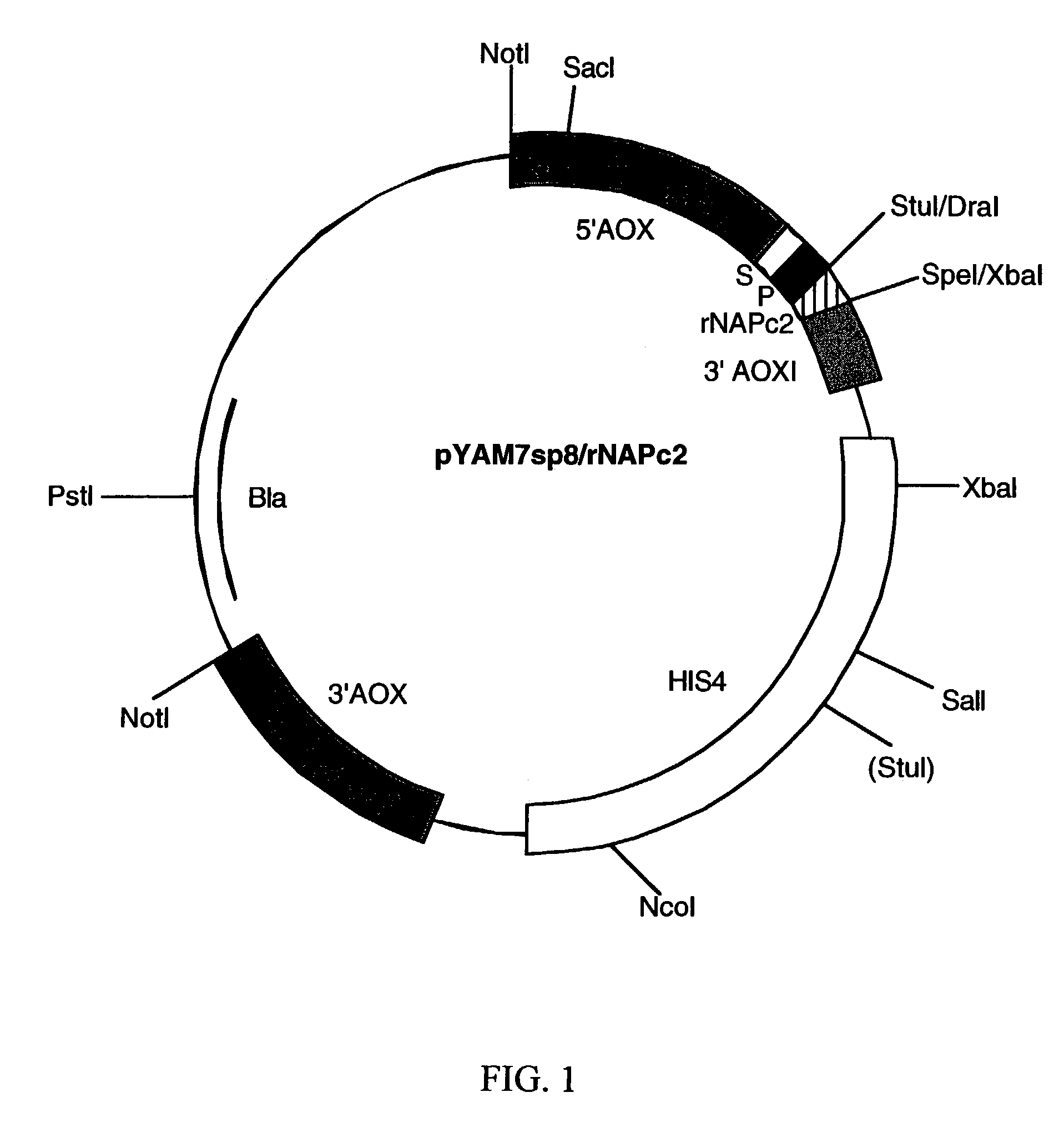
Method of treatment of hemorrhagic disease using a factor VIIa/tissue factor inhibitor
ActiveUS7132398B2Decrease or prevent said coagulopathy and/or inflammatory responseBiocidePeptide/protein ingredientsFactor VIIaNematode
The present invention provides methods of treating hemorrhagic fevers where selective inhibitors of fVIIa / TF are used as a treatment for hemorrhagic fevers and have therapeutic effects which include ameliorating and / or preventing coagulopathy and inflammatory responses. These inhibitors include certain proteins which are part of a family termed Nematode-Extracted Anticoagulant Proteins (“NAPs”). Other inhibitors include Tissue Factor Pathway Inhibitor (“TFPI”) and TFPI analogs.
Owner:DENDREON PHARMA LLC
Preparation and application of hemorrhagic fever associated pathogen identifying gene chip
ActiveCN105087824APracticalShort detection cycleNucleotide librariesMicrobiological testing/measurementOligonucleotide chipOligonucleotide
The invention relates to a hemorrhagic fever associated pathogen identifying gene chip; the preparation method comprises preparation of a specific primer, preparation of a pathogen specific oligonucleotide probe, preparation of an oligonucleotide chip, establishment of an RT-PCR (reverse transcription-polymerase chain reaction) system and establishment of a hybrid system and a signal detection method. The gene chip prepared by the invention can be used for simultaneously identifying 16 hemorrhagic fever associated pathogen microorganisms, including Zaire Ebola virus, Sudan Ebola virus, marburg virus, lassa virus, junin virus, Machupo virus, rift valley fever virus, Crimea-Congo hemorrhagic fever virus, plasmodium, hantaan virus, SFTS (severe fever with thrombocytopenia syndrome) virus, dengue virus, yellow fever virus, Chikungunya virus, influenza A virus and influenza B virus. The gene chip has the characteristics of being rapid and accurate, high in throughput and high in sensitivity; and a new technological means is offered for the diagnosis of hemorrhagic fever pathogen, health supervision and the control and prevention of infectious diseases.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
Method for quantitatively detecting hantaan virus load through one-step fluorescence probe real-time quantitative reverse transcription polymerase chain reaction
InactiveCN102634609AQuantitatively accurateStrong specificityMicrobiological testing/measurementMicroorganism based processesFluorescenceSyndrome patient
The invention discloses a method for quantitatively detecting hantaan virus load through one-step fluorescence probe real-time quantitative reverse transcription polymerase chain reaction. According to the method, the overall-length fragment of in vitro transcription S of the hantaan virus is utilized as a quantitative detection standard substance, a one-step fluorescence probe real-time quantitative reverse transcription polymerase chain reaction method is established by the design of detecting a virus gene sequence primer and a prober and the selection of the method so as to quantitatively detect the hantaan virus load of the blood plasma or other body fluid and tissues of a hemorrhagic fever with renal syndrome patient. Compared with a conventional PCR (Polymerase Chain Reaction) detection method, the method has the advantages that the specimen detection and operation steps are few, the sensitivity is high, the linear relation is good, and the experiment shows that the detection method has good specificity and repeatability and provides a powerful tool for the scientific research and preventive treatment of hemorrhagic fever with renal syndrome.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Murine original monoclonal antibody 3D8 identified hantaan virus glycoprotein neutralizing epitope peptide and application thereof
ActiveCN102731623ADirect determination of linear epitopesViral antigen ingredientsAntiviralsViral glycoproteinProtein target
The invention discloses a neutralizing monoclonal antibody 3D8 identified HTNV-GP specific B cell epitope and key amino acid residue sequence of the epitope for treating hemorrhagic fever with renal syndrome (HFRS) caused by hantaan virus (HTNV) infection. The B cell epitope has an amino acid sequence of 882GFLCPEFPGSFRKKC896. The epitope peptide can be applied to study of mechanism of 3D8 neutralizing monoclonal antibody in treatment of HFRS, or preparation of drug for treating HFRS and preparation of novel vaccine strain aiming at hantaan virus 76-118, or development of novel diagnostic kitfor hantaan virus 76-118 as a target protein. The invention has good prospects of development and application in the field of HFRS specific immunotherapy.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Use of anti-hantavirus medicament arbidol
InactiveCN101461805AInhibition of replicationInduces antiviral state effectsOrganic active ingredientsAntiviralsSide effectMortality rate
The invention discloses application of a medicine in resisting Hantaan viruses, namely arbidol. The arbidol has obvious effect of inhibiting the Hantaan viruses in vitro, and the antivirus effect of drug administration before the viruses enter a cell is stronger than that of the drug administration after the viruses enter the cell. The concrete embodiment comprises the following: the drug administration is performed before and after the infection, the positive rate of virus-infected cells and the fluorescence intensity are reduced along with the concentration increase, and the medicine has dosage effect; and the medicine can obviously reduce the positive rate of virus infection, and the mRNA expression of the viruses is reduced. The arbidol has protection and treatment effect on the infection of the Hantaan viruses on a suckling mouse, and the protection effect is stronger than the treatment effect. The drug administration is performed within 24h before the infection; and with the increase of the dosage of the medicine, the death rate of the mouse is reduced, and the average survival days are extended. The drug administration within 24h after the infection can not improve the survival rate of an animal, but can extend the average survival days of the animal. The arbidol has the effect of inhibiting the Hantaan viruses in a body of the animal. The drug administration within 24h before the infection can lighten the pathologic change of tissues (lung, kidney, and brain), and has treatment effect on HFRS. The medicine has prevention effect and also has treatment effect on patients with the hemorrhagic fever with renal syndrome (HFRS) caused by the Hantaan viruses, and no toxic side effect is found.
Owner:WUHAN UNIV
Recombinant vesicular stomatitis virus vaccines for viral hemorrhagic fevers
InactiveUS20060193872A1SsRNA viruses negative-senseSugar derivativesVesicular stomatitis virusVirology
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Protective metallothionein analog compounds, their compositions and use thereof in the treatment of pathogenic diseases
InactiveUS20160101079A1Enhancing cellular GSH levelReduce infectivityBiocidePeptide/protein ingredientsAntioxidantViral disease
Embodiments of the present invention relate generally the use of certain compositions, e.g., compositions comprising a glutathione precursor and a selenium source, in the therapy of viral diseases and / or reducing the incidence of viral diseases. Related embodiments of the present invention relate to treatment and / or reducing the incidence of respiratory ailments caused by respiratory syncytial virus (RSV) or hemorrhagic fever (EHF) caused by Ebola viruses (EBV) or Marburg virus. Yet in other embodiments, the invention relates to reducing metal toxicity in a biological system, which involves contacting the biological system with a composition comprising a glutathione precursor and a selenium source, optionally together with a chelating agent, an antioxidant, a metallothioneine protein or a fragment of metallothioneine.
Owner:CRUM ALBERT
Xinjiang hemorrhagic fever virus nucleoprotein antigen gene as well as recombinant protein and application thereof
The invention relates to the technical filed of antigen genes, in particular to a Xinjiang hemorrhagic fever virus nucleoprotein antigen gene (XHFNP235) as well as recombinant protein and application thereof. The gene has a nucleotide sequence as sequence 1. In the invention, the Xinjiang hemorrhagic fever virus nucleoprotein antigen gene (XHFNP235) and the recombinant protein thereof are obtained from Xinjiang hemorrhagic fever virus. Through PCR amplification, cloning, transformation, expression plasmid construction, induction expression and immunoassay detection, the Xinjiang hemorrhagic fever epitope is positioned at the 235th to 305th amino acid region of NP protein, and the region is a highly conservative amino acid region of the NP protein, which further shows that the XHFNP235 recombinant protein can be a candidate diagnosis antigen for Xinjiang hemorrhagic fever, and provides a new way for diagnosing and applying Xinjiang hemorrhagic fever.
Owner:THE CENT FOR DISEASE CONTROL & PREVENTION OF XINJIANG UYGUR AUTONOMOUS REGION
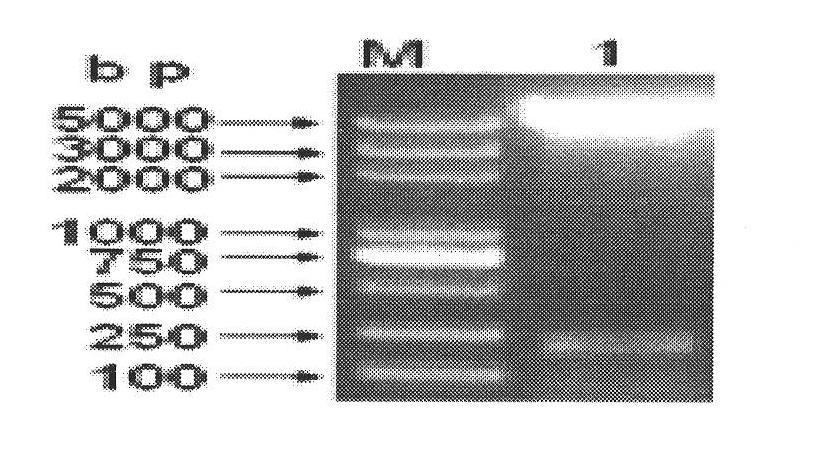
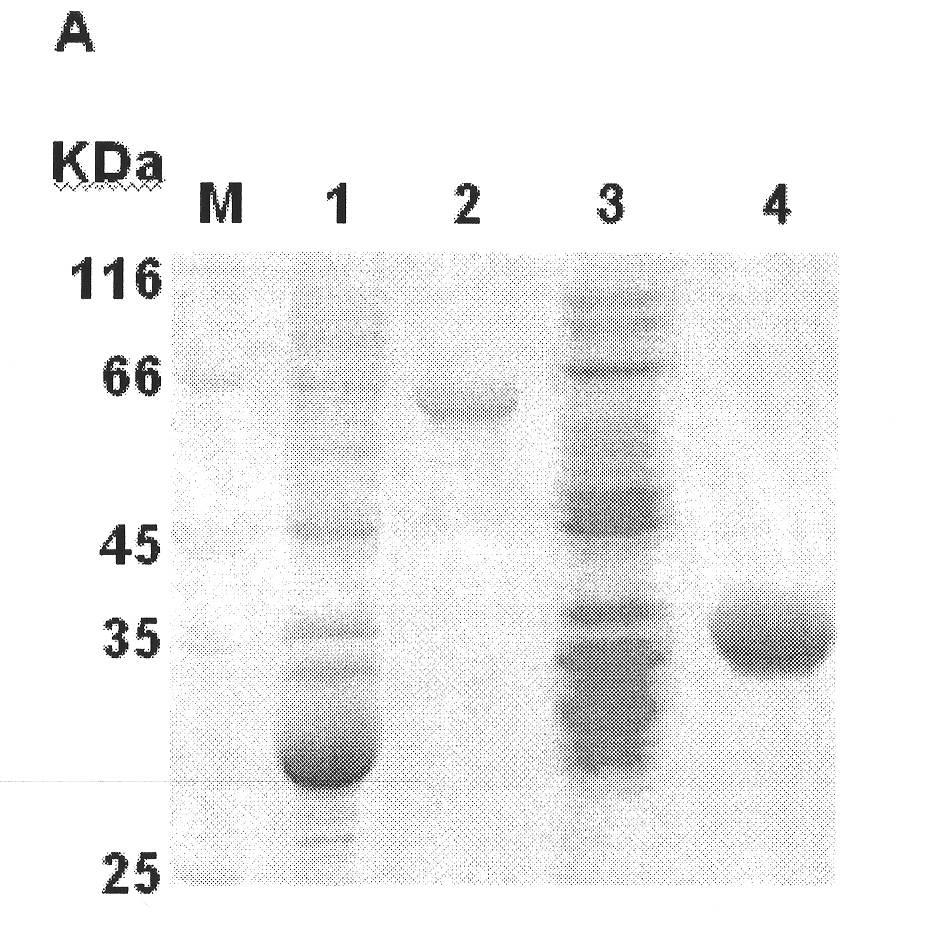
Kidney syndrome hemorrhagic fever Vero cell bivalent purified vaccine and industrialized producing process thereof
InactiveCN1990041ALow costEasy to operateViral antigen ingredientsAntiviralsPediatricsHemorrhagic fever virus
The invention involves nephrotic syndrome hemorrhagic fever Vero cells purified bivalent vaccine and its industrialized preparation methods. The nephrotic syndrome hemorrhagic fever Vero cells bivalent purified vaccine in the inventioncontains effective doses of nephrotic syndrome hemorrhagic fever deactive virus type I, type II and vaccine adjuvants, the type I and type II virus are from PS-6 (C-3) strains, CCTCC-V200503 and L99(C-2) strains, CCTCC-V200504 respectively. The preparation method of the invention includes: 1. Preparating the vaccine stock solution nephrotic syndrome hemorrhagic fever virus PS-6(C-3), CCTCC-V200503 and L99(C-2),CCTCC-V200504, inactivating virus with formaldehyde; 2. combining the deactive vaccine stock solution of PS-6(C-3), CCTCC-V200503 and L99(C-2),CCTCC-V200504 and purifying, the process contains centrifugal separation, hyperfiltration, concentration, zinc acetate deposition and column chromatography; 3. adding adjuvant into the purified vaccine stock solution and getting the purified bivalent vaccine. The nephrotic syndrome hemorrhagic fever Vero cells purified bivalent vaccine in the invention is suitable for the prevention of nephrotic syndrome hemorrhagic fever mainly based on all type I and type II.
Owner:长春生物制品研究所有限责任公司
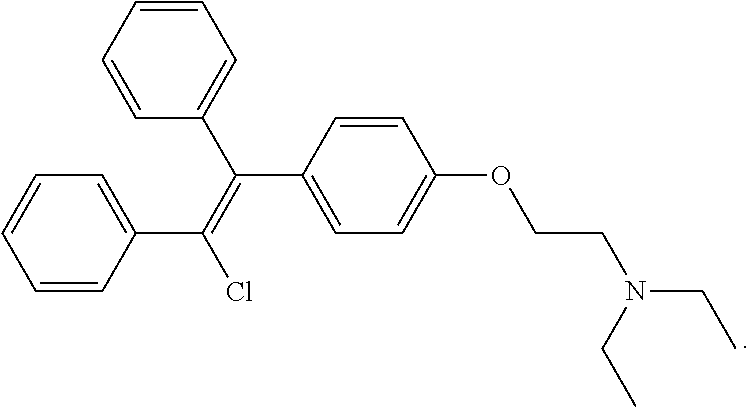
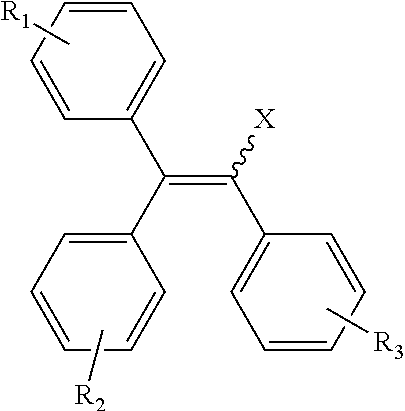
Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain novel semicarbazides, sulfonyl carbazides, ureas and related compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by hemorrhagic fever viruses is disclosed, i.e., including but not limited to Arenaviridae (Junin, Machupo, Guanavito, Sabia and Lassa), Filoviridae (ebola and Marburg viruses), Flaviviridae (yellow fever, omsk hemorrhagic fever and Kyasanur Forest disease viruses), and Bunyaviridae (Rift Valley fever).
Owner:KINETA FOUR LLC
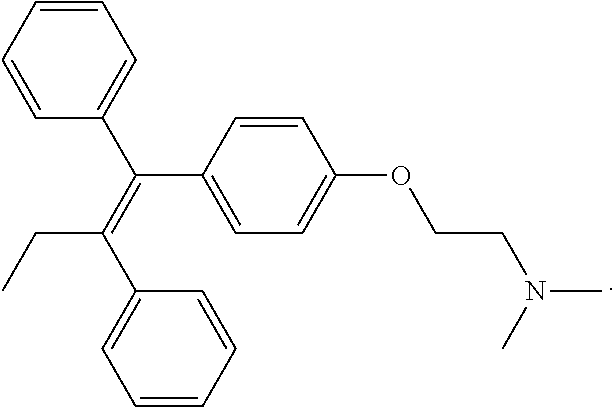
Antiviral drugs for treatment of arenavirus infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain novel compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by hemorrhagic fever viruses is disclosed, i.e., including but not limited to, Arenaviridae (Junin, Machupo, Guanarito, Sabia, Lassa, Tacaribe, Pichinde, and LCMV), Filoviridae (Ebola and Marburg viruses), Flaviviridae (yellow fever, Omsk hemorrhagic fever and Kyasanur Forest disease viruses), and Bunyaviridae (Rift Valley fever and Crimean-Congo hemorrhagic fever).
Owner:KINETA FOUR LLC
Adenovirus high expression vector for improving expression of hantavirus fusion protein G1S0.7
InactiveCN101812478AHigh expressionImprove expression levelFermentationVector-based foreign material introductionForeign proteinTransfer vector
The invention relates to reconstruction of an adenovirus transfer vector, in particular to reconstruction of a transfer vector of mosaic gene G1S0.7 of hantavirus (HV) serving as hemorrhagic fever with renal syndrome (HFRS) pathogen for improving the expression of hantavirus fusion protein G1S0.7. The adenovirus transfer vector G1S0.7-pShuttle containing the mosaic gene G1S0.7 is reconstructed by using the gene recombination technology. The reconstruction comprises the following steps: replacing a CAG promoter / enhancer for a CMV promoter; or inserting a WPRE transcriptional control element at the 3' end of the promoter; or replacing the promoter and inserting a WPRE control element. The vector expresses the fusion proteins G1S0.7 respectively, and compares the expression level of foreign protein in each recombinant adenovirus. The result shows that the vector can effectively improve the expression of the fusion protein G1S0.7 compared with the primary transfer vector pShuttle, and has most obvious effect of replacing a promoter group.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Organic tea of TeiGuanYin tea containing nano-selenium, picking and preparing method
InactiveCN101401600AMeets Organic Tea StandardsStandards compliantPre-extraction tea treatmentFood preparationKeshan diseaseArthritis
The invention aims to provide a TeiGuanYin organic tea leaf containing nano-selenium and a method for picking the same. The TeiGuanYin organic tea leaf containing nano-selenium contains 40 to 65 mg of the nano-selenium per 100g, the nano-selenium is between 50 and 200 nm and the balance being dietary fibers, carbohydrates, proteins, microelements and vitamins A, A1, B1, B2, B5, E, renieratene and elements such as manganese, magnesium, phosphorus, zinc, iron, copper, sodium, calcium, potassium, thereby conforming to the standard of organic tea. The selenium is a necessary element of the human body and has functions of eliminating free radicals, preventing cancer, caring beauty and delaying aging, losing weight, resisting radiation, regulating immunity and treating the Keshan disease and so on, and is a nutritional anti-cancer agent recognized in the medical field. The frequent drinking of the nano-selenium TeiGuanYin organic tea has better effect to prevent various diseases such as hepatitis, arthritis, cataract and cardiovascular disease hemorrhagic fever and contributes to health and longevity for the middle-aged and the old.
Owner:张金松
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com