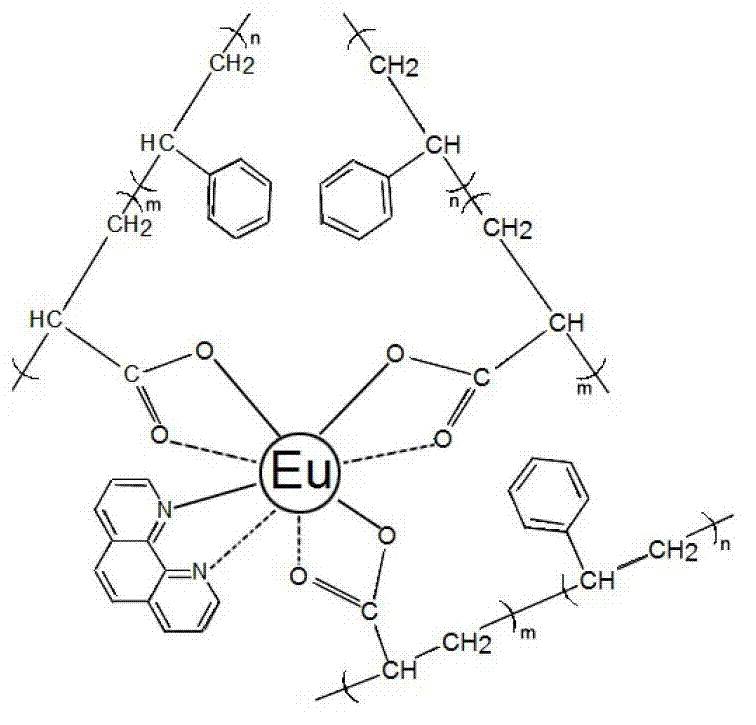
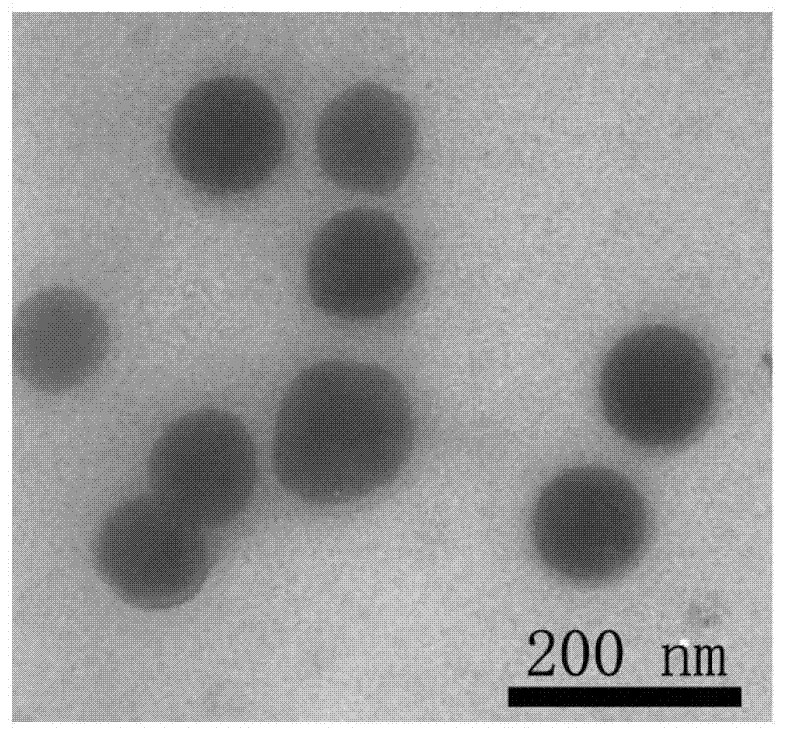
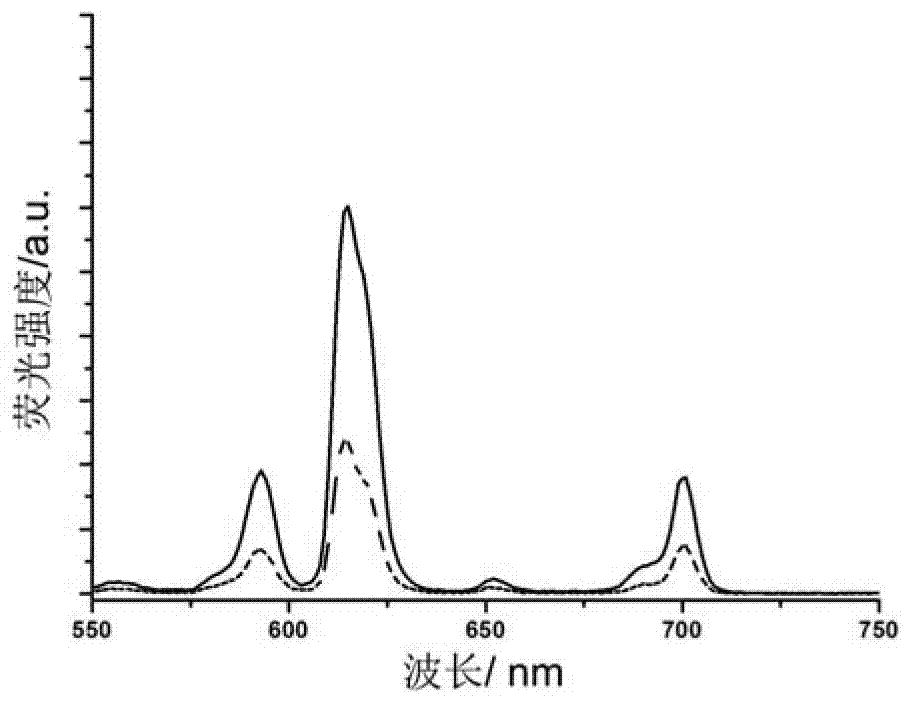
Preparation method of polymer-rare earth ion luminous micelles
A technology of rare earth ions and polymers, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of inability to uniformly disperse rare earth ions, restrict material molding and processing, and limit the application of composite materials, etc., to achieve stable product structure and reliable principle , a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In this example, AB-type styrene-b-acrylic acid diblock copolymers were first synthesized by the reversible addition-fragmentation chain transfer method: 1.612 g of styrene monomer and chain transfer agent were measured according to the molar ratio of 500:5:1. (trithioester C 16 h 19 o 2 NS 3) 0.055g and azobisisobutyronitrile initiator 0.005g, add 5ml of dioxane as a solvent, put the above materials into a round bottom flask and seal it, and react in a constant temperature oil bath at 60°C after passing nitrogen gas for about 30 minutes After 10 hours, after the reaction, the reaction mixture was dropped into about 150ml of ether to precipitate the polymer and vacuum-dried; Mix isobutyronitrile initiators, use 15ml of dioxane as a solvent, pass nitrogen gas for 30 minutes, and react in a constant temperature oil bath at 80°C for 6 hours. After the reaction, the reaction solution is precipitated with 200ml of ether to obtain styrene-b-acrylic acid Block copolymer; D...
Embodiment 2
[0022] This embodiment obtains the styrene-b-acrylic acid block copolymer solution of 0.1mol / L molar concentration of acrylic acid unit according to Example 1; the preparation of its polymer-terbium ion micelles is according to the ratio of acrylic acid unit / terbium ion 1:1 Molar ratio measures 5ml of polymer solution and 0.1mol / L terbium chloride solution 5ml respectively, and adds 0.099g of o-phenanthroline of the same molar amount as terbium chloride as the second ligand; the three reactants are mixed Place in a three-neck flask with a condensing reflux device, and react for 5 hours in a constant temperature oil bath environment at 50°C to obtain a solution of terbium ion-coordinated polymer micelles.
Embodiment 3
[0024] The synthesis of the styrene-b-(4-vinylpyridine) block copolymer involved in this embodiment is to measure 1.612g of styrene monomer according to the ratio of 500:5:1, chain transfer agent (trithioester C 16 h 19 o 2 NS 3 ) 0.055g and azobisisobutyronitrile initiator 0.005g, add 5ml of dioxane as a solvent, put the above reagents into a round bottom flask and seal it, and react in a constant temperature oil bath at 60°C after passing nitrogen gas for about 30 minutes After 10 hours, after the reaction, the reaction mixture was dripped into about 150ml of ether to precipitate the polymer and vacuum-dried; Azobisisobutyronitrile initiators were mixed, and 10ml of dioxane was used as a solvent, and nitrogen gas was passed for 30 minutes, and then reacted in a constant temperature oil bath at 80°C for 6 hours; after the reaction, the mixed solution was precipitated with 200ml of ether to obtain styrene- b-(4-vinylpyridine) block copolymer; use nuclear magnetic resonance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com