Anti-African swine fever virus p54 protein monoclonal antibody and preparation method and application thereof
A technology of African swine fever virus and monoclonal antibody, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Selection and preparation of immunogens
[0049] The viral structural protein p54 is encoded by the E183L gene and is located in the inner membrane of the viral envelope. The N-terminus of p54 is relatively conserved among virus strains and contains a unique cysteine. It is used in the process of virus morphogenesis, activity, and invasion. have an important role. In the previous experiment, a peptide was synthesized based on the NTD sequence of the p54 protein to simulate NTD. The peptide had a good reaction with the serum of ASFV-infected clinically recovered pigs, which proved that the NTD region of the p54 protein contained antigenic determinants, which could induce the body to produce antibodies against NTD. Therefore, the present invention uses p54 protein NTD coupled to carrier protein BSA as an immunogen. Specifically, the preparation of the immunogen includes the following steps:
[0050] (1) With reference to the p54 protein sequence of ASFV strain...
Embodiment 2
[0053] Example 2 Preparation of monoclonal antibodies
[0054] 1. Animal Immunization
[0055] (1) Freund's complete adjuvant is added to the immunogen NTD-BSA, and it is used for the first immunization after being emulsified;
[0056] (2) 2 female BALB / c mice aged 4-8 weeks were immunized by subcutaneous injection at multiple points on the back, and the immunization dose was 10 μg / mice;
[0057] (3) BALB / c mice were boosted with the same method and dose after emulsification with incomplete Freund's adjuvant and immunizing antigen every 3 weeks;
[0058] (4) After three weeks, the tail vein blood was collected to measure the specific antibody titer against NTD, and the mice with higher titer were selected ( figure 1 ), 3 to 4 days before cell fusion, BALB / c mice were super-immunized with immunogen without adjuvant by tail vein injection, and the immunization dose was 20 μg / mice.
[0059] 2. Cell fusion and monoclonal antibody preparation
[0060] The spleen cells of the im...
Embodiment 3
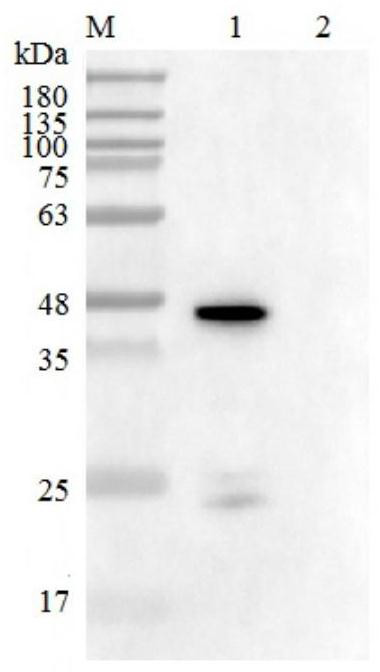
[0076] Example 3 Purification and identification of antibodies
[0077] 1. Purify antibody by saturated ammonium sulfate precipitation method, the operation method is as follows:
[0078] (1) Take 5ml of monoclonal antibody ascites, add 5ml of PBS buffer, and then add 2.5ml of saturated ammonium sulfate solution dropwise to make a final concentration of 20% ammonium sulfate solution, stir while adding, mix well, and let stand 30min.
[0079] (2) 8000r / min, centrifuge for 20min, discard the precipitate to remove fibrin.
[0080] (3) Add 12.5 ml of saturated ammonium sulfate solution to the supernatant, mix well, and let stand for 30 minutes.
[0081] (4) Centrifuge at 8000 r / min for 20 min, and discard the supernatant.
[0082] (5) Add 10 ml of PBS buffer to the precipitate to dissolve it, then add 5 ml of saturated ammonium sulfate solution to make it a 33% ammonium sulfate solution, mix well, and let it stand for 30 minutes.
[0083] (6) Centrifuge at 8000 r / min for 20 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com