Stable lyophilized preparation of recombinant human anti-CD20 monoclonal antibody
A monoclonal antibody, freeze-dried preparation technology, applied in the direction of antibody, freeze-dried delivery, drug combination, etc., to achieve the effect of reducing degradation and aggregation, improving stability, and rapid reconstitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Prepare the solution before freeze-drying with the concentration shown in Table 1, and freeze-dry it into a finished product.
[0025] Table 1
[0026] Element
concentration
Recombinant Human Anti-CD20 Monoclonal Antibody
20mg / mL
Histidine-Histidine Hydrochloride
20mM
20mg / ml
25mg / ml
Polysorbate 80
0.05%
0.02mg / ml
2mg / ml
PH value
5.7
[0027] The purified recombinant human anti-CD20 monoclonal antibody stock solution (concentration 10-30 mg / mL, 20 mM citrate buffer, pH 6.6) was concentrated by ultrafiltration with an ultrafiltration membrane bag (molecular weight cut-off 30 kDa), and ultrafiltered to In 20mM, pH5.7 histidine-histidine hydrochloride buffer, the final concentration of ultrafiltration is (20-30mg / mL), and then mixed with a certain proportion of sucrose, mannitol, polysorbate 80, edetic...
Embodiment 2
[0033] Embodiment 2: Prepare the solution before freeze-drying with the concentration shown in Table 2, and freeze-dry it into a finished product.
[0034] Table 2
[0035] Element
concentration
Recombinant Human Anti-CD20 Monoclonal Antibody
20mg / mL
Histidine-Histidine Hydrochloride
20mM
20mg / ml
30mg / ml
Polysorbate 20
0.05%
0.02mg / ml
1mg / ml
PH value
5.5
[0036] The purified recombinant human anti-CD20 monoclonal antibody stock solution (concentration 10-30 mg / mL, 20 mM citrate buffer, pH 6.6) was concentrated by ultrafiltration with an ultrafiltration membrane bag (molecular weight cut-off 30 kDa), and ultrafiltered to 20mM, pH5.5 histidine-histidine hydrochloride buffer solution, the final concentration of ultrafiltration is (20-30mg / mL), and then mixed with a certain proportion of trehalose, mannitol, polyso...
Embodiment 3
[0038] Embodiment 3: Stability investigation
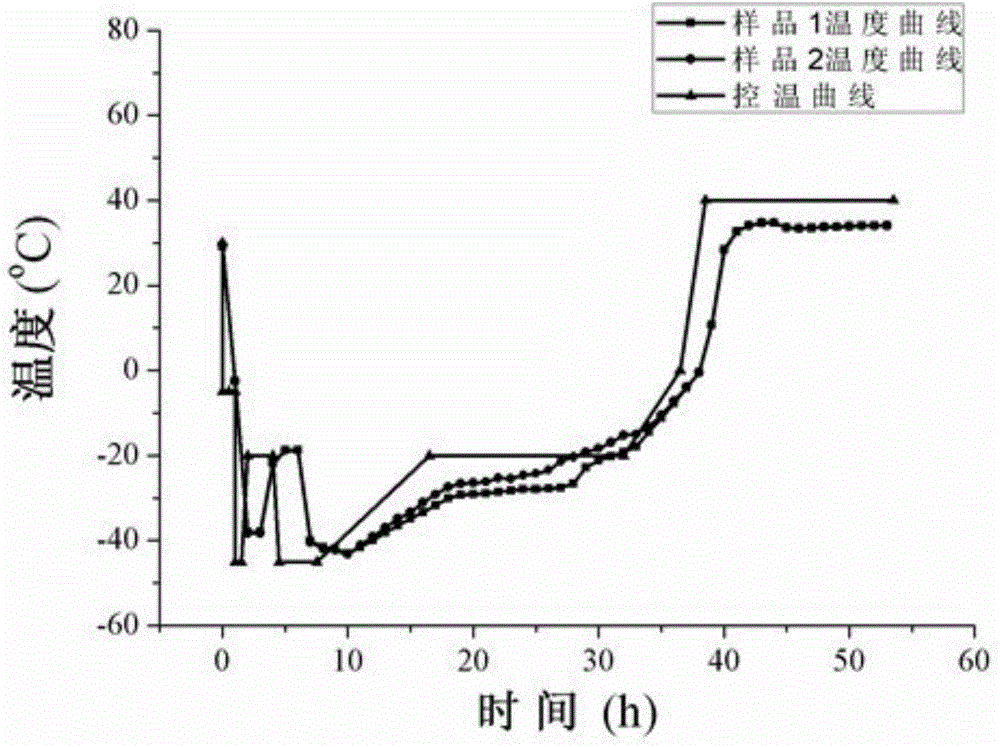
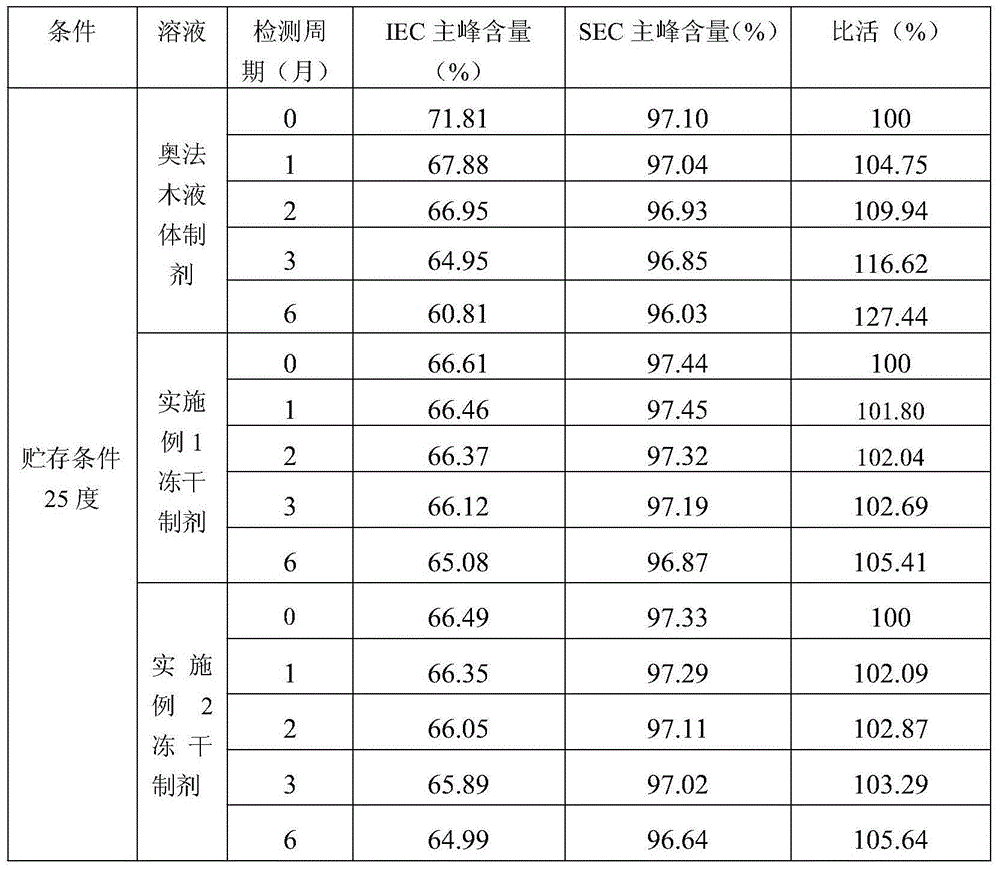
[0039] The stability of the above freeze-dried preparation was investigated by experiment of influencing factors under high temperature and light conditions, and compared with the liquid preparation (ofatumumab injection) under the same conditions. The experimental conditions and methods are as follows: (1) Accelerated experiment at 25°C: place samples in a thermostat at 25°C, and take samples in October, January, February, March, and June; (2) Accelerated experiment at 40°C: Place samples in a 40°C incubator, and take samples in 0 months, January, February, and March; (3) 60°C accelerated experiment: place samples in a 60°C incubator, and take samples in 0 and 10 days; ( 4) Illumination experiment: Place samples in a room temperature light box at 5000±500 lx, and take samples on day 0, day 5, and day 10. The sampled freeze-dried preparation was reconstituted with 5ml of water for injection. After reconstitution, the test was pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com