Photosensitizers and MRI enhancers
A diagnostic agent, the technology of SARS virus, which is used in the treatment of the above diseases, can solve the problems of inefficiency, high toxicity, chemical and photochemical instability of photosensitizers in photoactive treatment of tumor cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173]Ammonia is added to the water until the pH of the solution is not less than 9. Chlorin e6 (0.1 g) was dissolved in the aqueous solution. An equimolar amount of zinc acetate (0.22 g) was added, and the reaction mixture was stirred at about 20°C for 15 minutes to obtain a complex formation reaction. The process and completion of the complex formation reaction are monitored by a spectrophotometer. Upon completion of the complex formation reaction, human serum albumin (SHA) (71 g) was added as a fixative to the reaction mixture. Once the immobilization reaction is completed (monitored by a spectrophotometer), the reaction product, the Zn-chlorin-e6 complex immobilized on the SHA, is purified by dialysis.
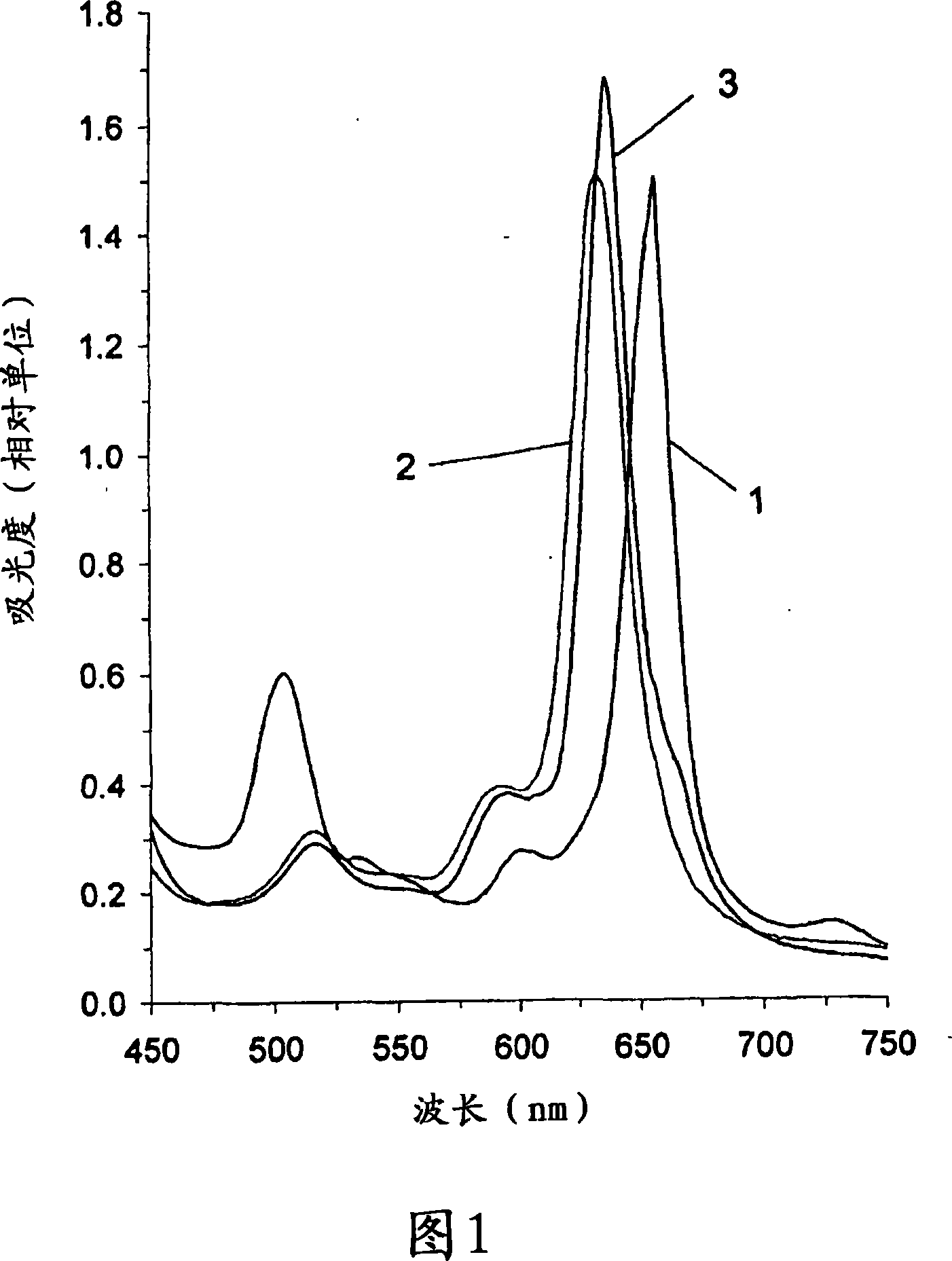
[0174] Figure 1 shows the (1) raw material chlorin e6 (λ max =656nm), (2) Zn-chlorin e6 complex (λ max =632nm) and (3) Zn-chlorin e6 complex immobilized on SHA (λ max =636nm) in the long-wave region of the visible light absorption spectrum.
[0175] Figure 1 shows that the for...
Embodiment 2
[0178] The synthesis process of the immobilized Zn-chlorin-e6 complex was carried out as shown in Example 1, but polyvinylpyrrolidone (PVP) (62g) was used as the immobilizer instead of human serum albumin (SHA).
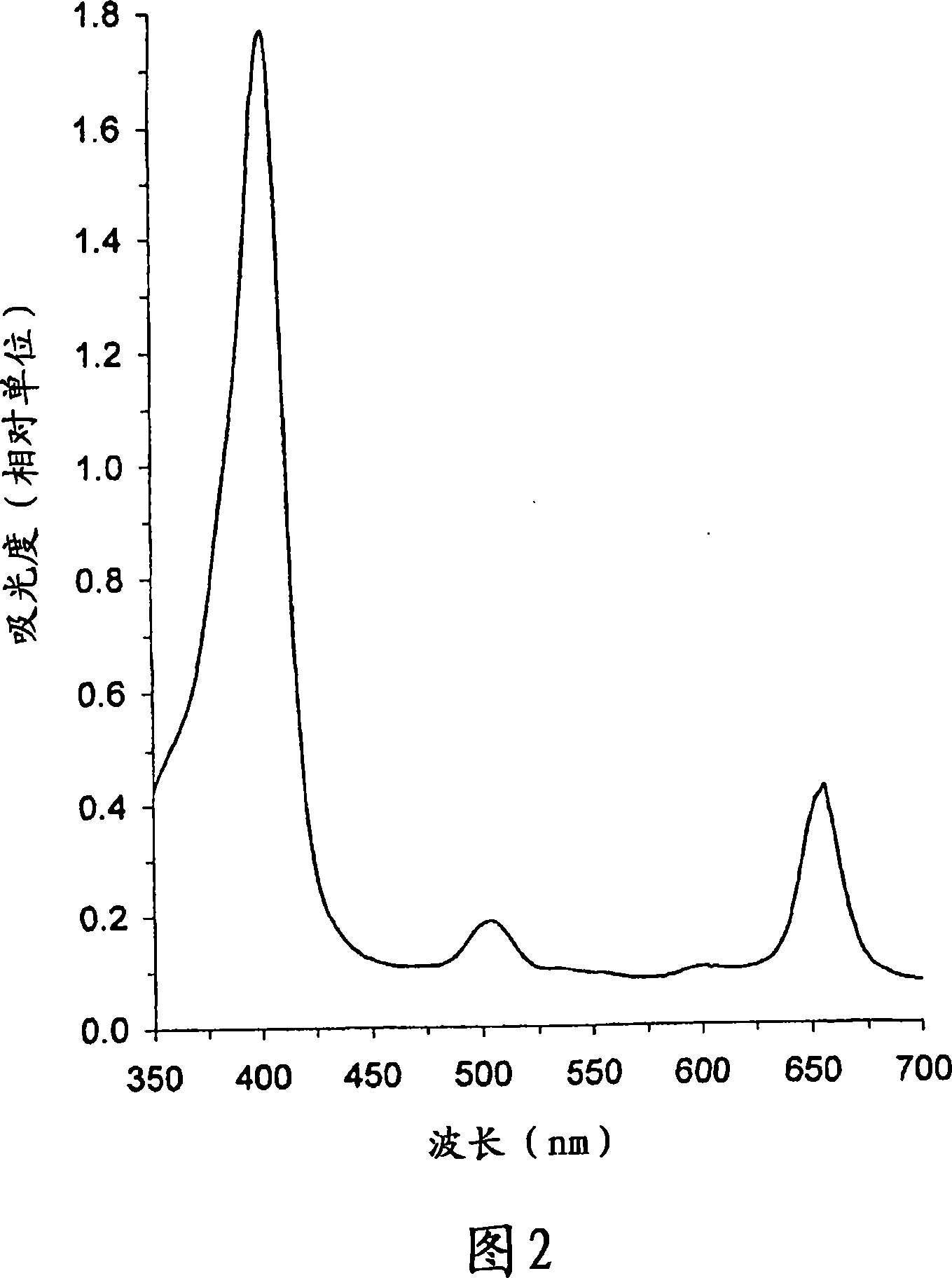
[0179] As shown in Figure 4, (1) the raw material Chlorin-e6 (λ max =656nm), (2) Zn-chlorin e6 complex (λ max =632nm) and (3) Zn-chlorin e6 complex immobilized on PVP (λ max =638nm) is actually the same as the visible absorption spectrum shown in FIG. 1. When the metal complex is formed, an obvious 24nm shortwave shift of the long wave peak is observed, and a small 6nm longwave shift when immobilized on the polymer PVP. When the Zn-chlorin e6 complex is formed, the chlorin-e6 is at λ max The moderate intensity peak at 502nm almost disappeared. All these changes indicate the completion of the reaction and the purity and homogeneity of the product obtained.
Embodiment 3
[0181] Ammonia is added to the water until the pH of the solution is not lower than 9. Then, chlorin e6 (1.0 g) was dissolved in the aqueous solution, an equimolar amount of SHA (71 g) was added, and the reaction mixture was stirred at about 20° C. for 17 minutes to immobilize the chlorin e6 on the SHA. Then an equimolar amount of zinc acetate (0.22 g) was added, and the reaction mixture was stirred at room temperature to complex the zinc with chlorin e6, which was monitored by a spectrophotometer. The reaction product Zn-chlorin-e6 complex immobilized on SHA was purified by dialysis.
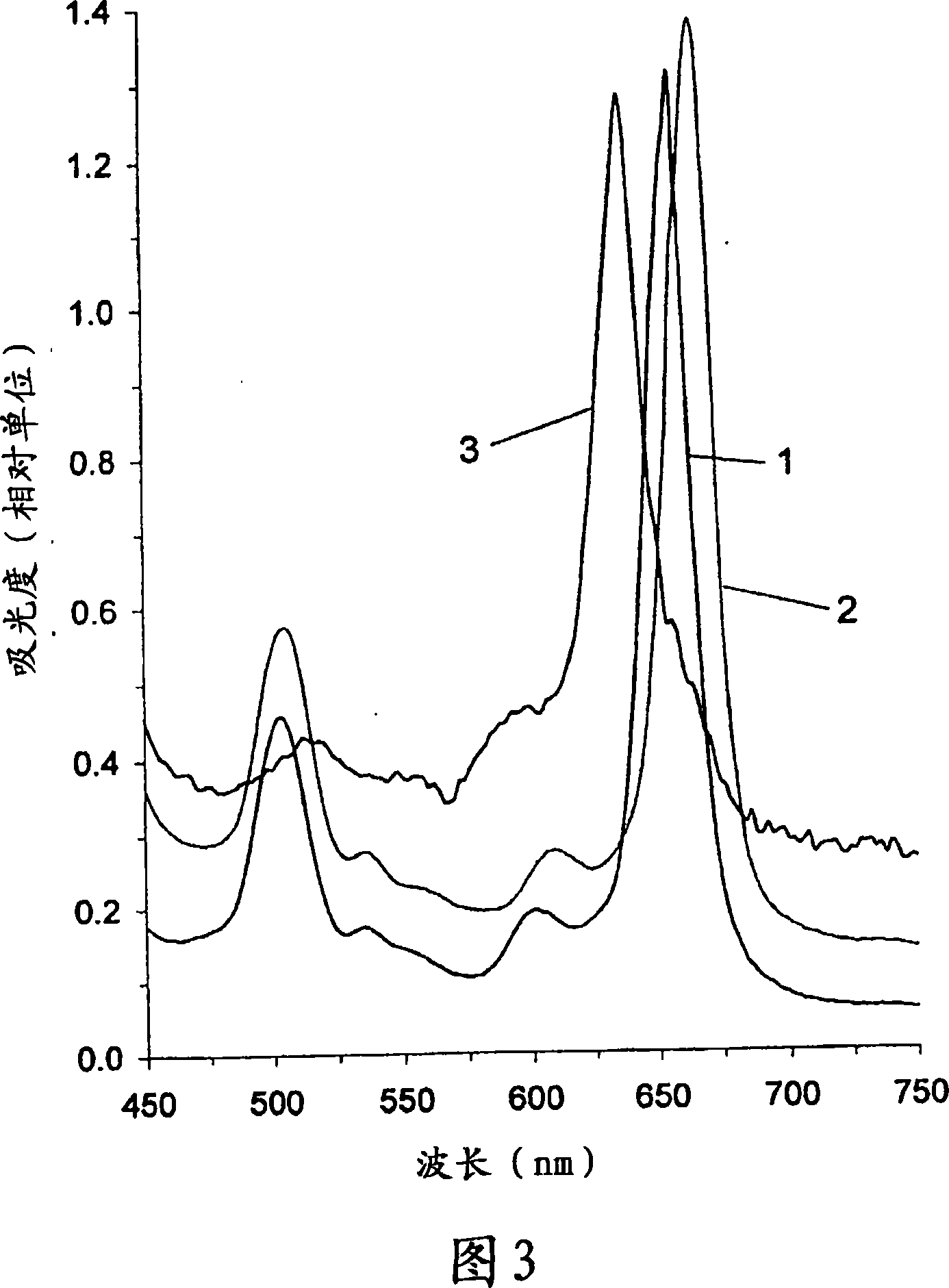
[0182] Figure 3 shows (1) the raw material chlorin-e6(λ max =656nm), (2) Chlorin e6(λ max =662nm) and (3) Zn-chlorin e6 complex immobilized on SHA (λ max =636nm) in the long-wave region of the visible absorption spectrum. Different from the first synthesis method (see Example 1), when the chlorin-e6 immobilized on the protein is formed, the 6nm long-wave shift of the absorption peak first appears, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com