Feline vaccine compositions and method for preventing chlamydia infections or diseases using the same
a vaccine composition and composition technology, applied in the field of inactivated feline chlamydia vaccine composition, can solve the problems of fpn being a major problem, inability to detect chlamydia, and inability to detect chlamydia, and achieve the effect of high antigen load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
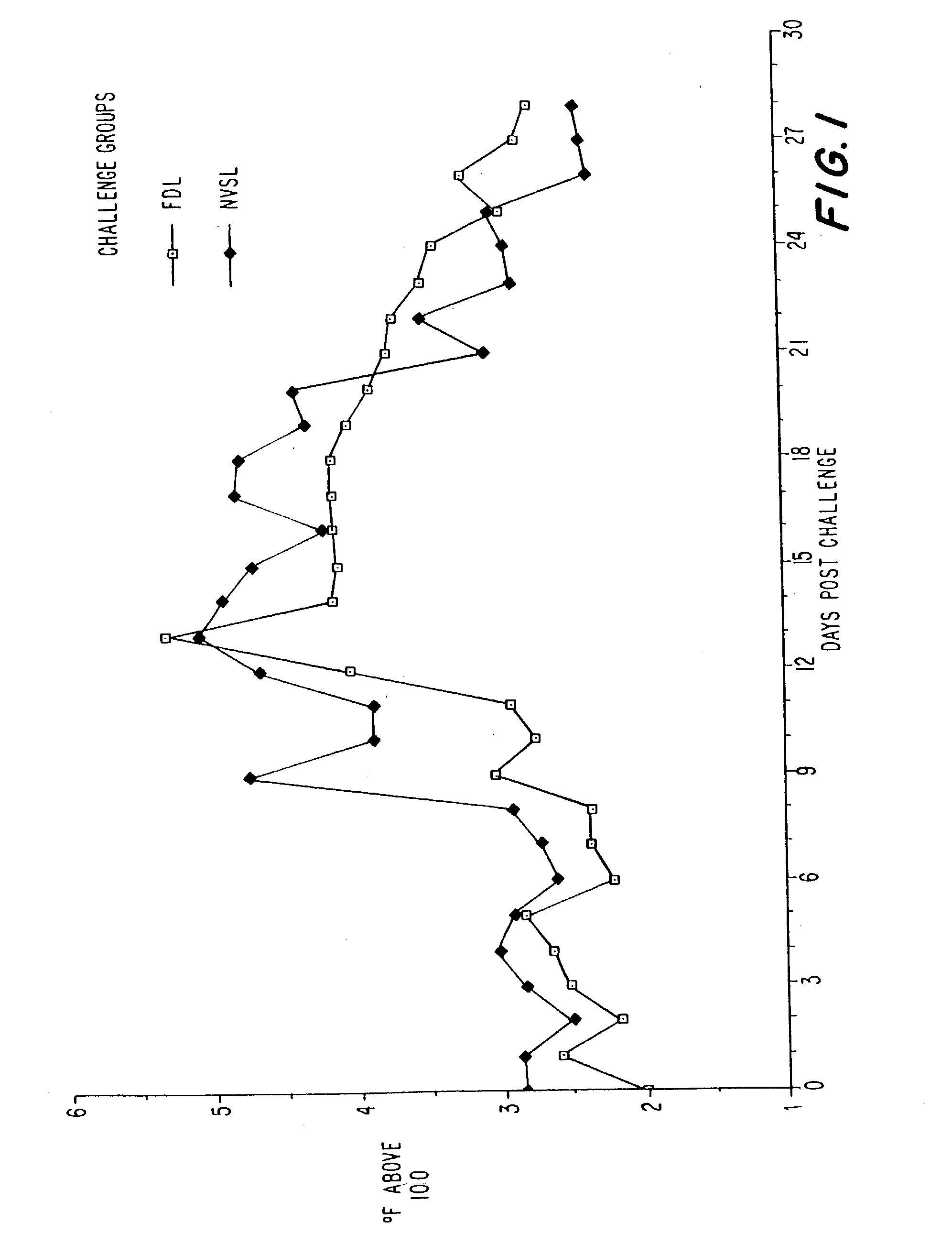
[0061] Challenge and Isolation of C. psittaci in Felines
[0062] Two challenge preparations were evaluated in young cats in order to produce consistent disease due to infection with Chlamydia psittaci.
[0063] A. Experimental Animals
[0064] Ten animals used for this experiment were specific pathogen free (SPF) cats purchased from Liberty Laboratories (Liberty Corner, N.J.). The cats were screened after receipt of antibodies to C. psittaci utilizing an ELISA assay for C. psittaci antibodies. The SPF cats were 10 to 12 weeks of age at the time of vaccination and approximately 16 to 18 weeks old at the time of challenge.
[0065] B. Experimental Design
[0066] Two 1 mL vaccinations of the FCP 1 and FCP 2 vaccines or fractional dose vaccines were administered intramuscularly 21 days apart. Two doses of Solvay's modified-live Eclipse.RTM.4 vaccine were given 21 days apart according to the label directions. Animals receiving the modified live vaccine were held in separate facilities both before and...
example 3
[0089] Correlation of Immunogenicity and Potency of FCP Vaccines Containing Saponin / AlPO.sub.4 as Adjuvants
[0090] A total of 91 SPF cats, 10 to 12 weeks of age, were utilized in this study. The FCP 1A vaccination group consisted of 21 cats. The FCP 1B and FCP 2A vaccination groups consisted of 20 cats each. The FCP 2B and the Solvay Eclipse.RTM.4 vaccination groups included 11 cats each. The non-vaccinated control group consisted of 11 cats. The reagent used to challenge the cats in the immunogenicity trials was a combination of the NVSL challenge preparation and the low egg passage preparation diluted to 10.sup.5.79 FLD.sub.50 titer per cat. Results indicate a reliable and accurate reproduction of disease from the use of the combined low egg passage preparation and NVSL challenge preparation.
[0091] A. Reduction in Fever in Vaccinated Cats as Compared to Controls Following Challenge
[0092] The temperature response of each animal in the five vaccine and control groups were measured. T...
example 4
[0104] Post Challenge Evaluation of Felines Vaccinated with FCP Compositions Containing EMA / NEOCRYL.RTM. / MVP Adjuvant System
[0105] The efficacy of FCP vaccines containing EMA / Neocryl.RTM. / MVP as adjuvants was evaluated in young cats via vaccination and challenge studies. The vaccines were administered intramuscularly or subcutaneously in two doses, 21 days apart. Efficacy of the FCP vaccines was demonstrated by challenging the vaccinated groups and age-match controls with the low egg passage virulent pneumonitis preparation as described below.
[0106] A total of 100 healthy, specific pathogen free (SPF) cats were purchased for the FCP vaccine efficacy testing. Ninety-five (95) cats were purchased from Liberty Laboratories (Liberty Crossing, N.J.) and five (5) cats were acquired from Harlan-Spraque Dawley (Indianapolis, Ind.). All test animals were approximately 16 to 20 weeks of age at the time of vaccination and approximately 20 to 25 weeks of age at the time of challenge. These anim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com