Vaccines against chlamydial infection
A technology for chlamydia and chlamydia protein, which is applied in the field of vaccines against chlamydia infection, and can solve problems such as infection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
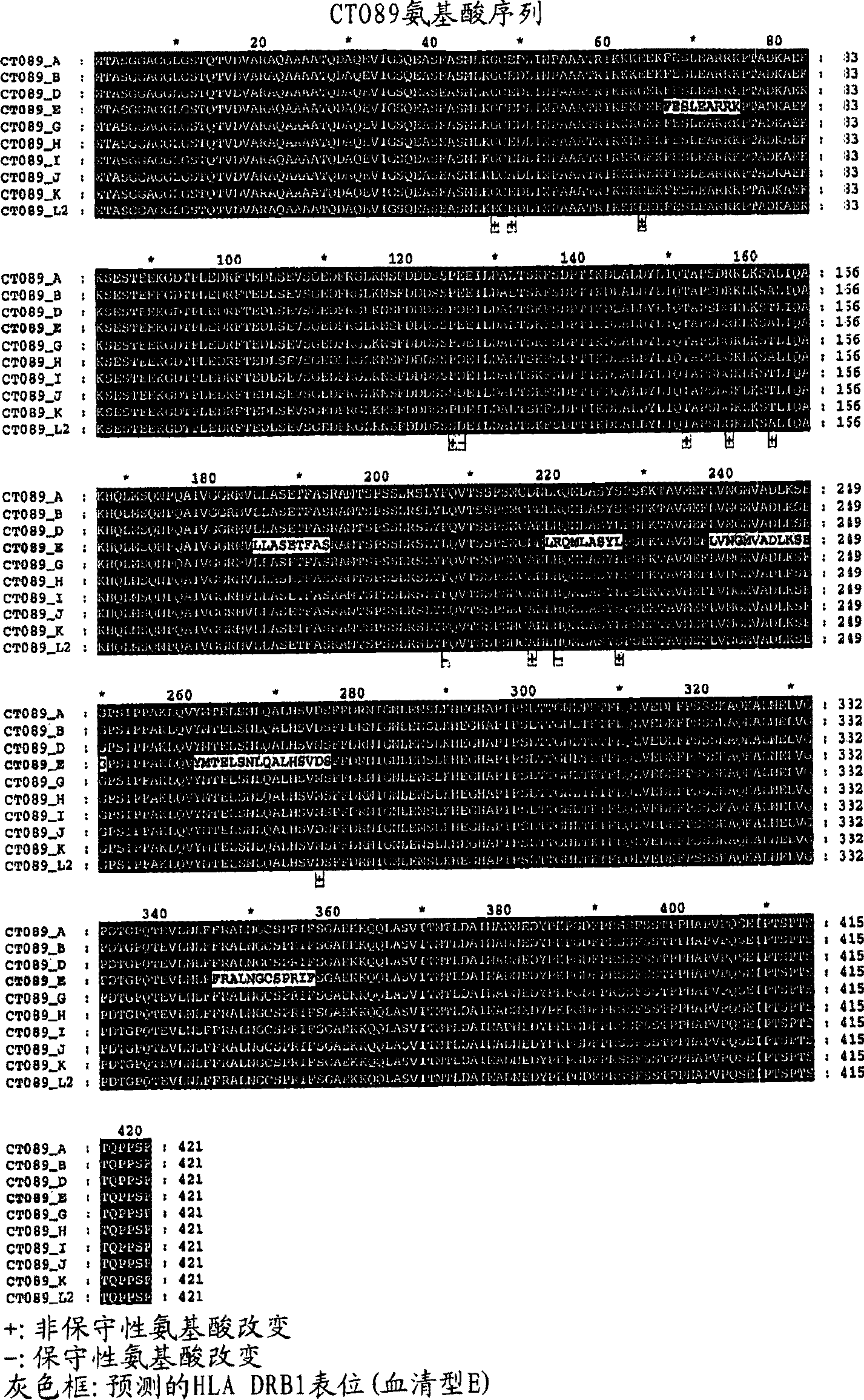
[0489] Example 1: Expression and purification of Chlamydia trachomatis recombinant protein
[0490] Several C. trachomatis genes were cloned into plasmids incorporating a 6X histidine tag at the N-terminus to allow expression and purification of recombinant proteins.
[0491] Two full-length recombinant proteins, Ct-622 and Ct-875, were expressed in E. coli. CtL2 and CtE expression screens were used to identify these two genes and express serotype E homologues. Primers used to amplify these genes were based on serotype L2 / E sequences. Genes were amplified using serotype E genomic DNA as a template. Once amplified, the fragment was cloned into pET-17b with an N-terminal 6X-His tag. After transformation of the recombinant plasmids into XL-I blue cells, DNA was prepared and the clones were fully sequenced. The DNA was then transformed into the expression host BL21-pLysS (Novagen) for recombinant protein production. Proteins were induced with IPTG and purified on Ni-NTA agaro...
Embodiment 2
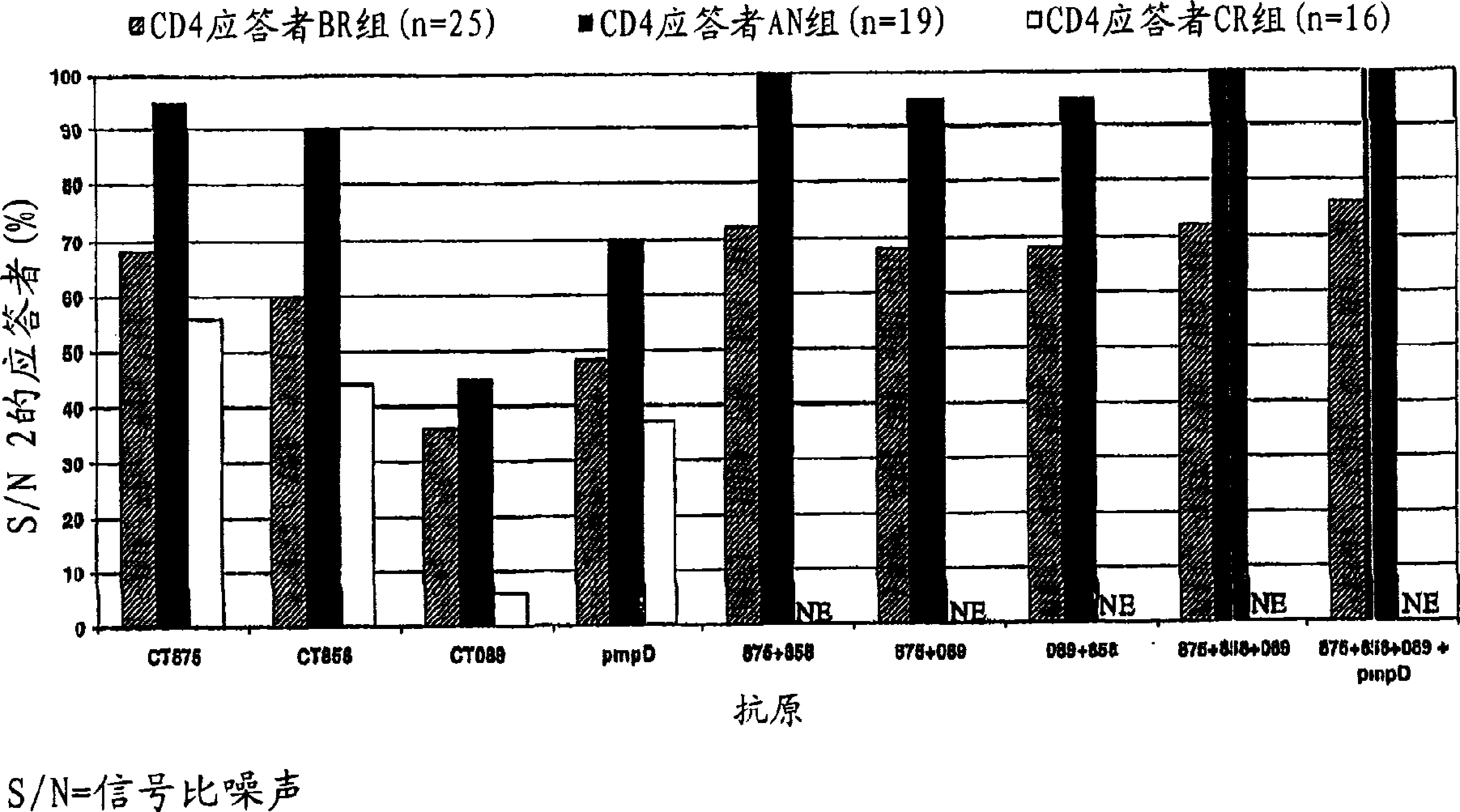
[0497] Example 2: Formulation of five different Chlamydia trachomatis antigen combinations and adjuvants
[0498] The antigen combinations in the table below were prepared as follows. 5 μg of each antigen was mixed in 50 μl PBS and then mixed with 50 μl AS01B adjuvant comprising 3D-MPL and QS21 formulated with cholesterol-containing liposomes to a total volume of 100 μl per dose.
[0499] After mixing with the antigen, the final composition of the adjuvant is:
[0500]
[0501]
Embodiment 3
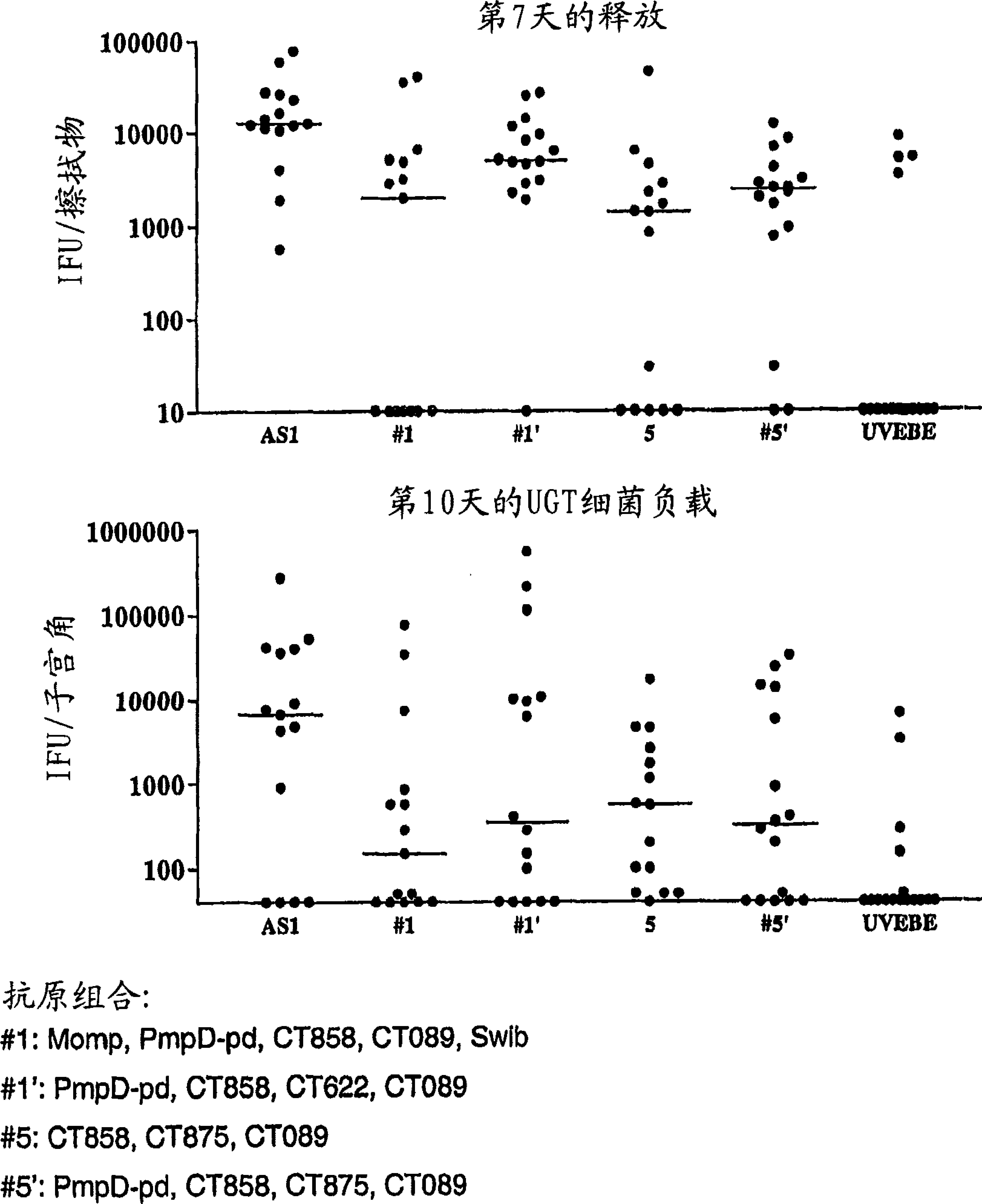
[0502] Example 3: Testing of Combinations of Chlamydia Trachomatis Antigens in a Mouse Model - Immunization Against Chlamydia Genital Tract Infection
[0503] This example demonstrates that vaccination with the combination of Chlamydia antigens described in Example 2 can significantly protect mice from Chlamydia infection.
[0504] A murine model of genital tract infection by human Chlamydia trachomatis (Ct) serotype K strains was developed that closely resembles the mechanism of infection in humans. This model was used to evaluate the effectiveness of immunizing mice with various combinations of Ct-specific antigens from different serotypes. Specifically, Balb / c mice and C57Bl / 6 mice were inoculated with the formulations of the adjuvant combinations described in Example 2. This model was also tried with a third mouse strain, DBA, but this model was not immune to Ct challenge, which did not occur in the positive control (UV-irradiated Chlamydia elegans (UVEB) formulated in AS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com