Application method of nafamostat mesylate to treatment of chlamydia infection of genital tract
A technology for nafamostat mesylate and reproductive tract infection, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
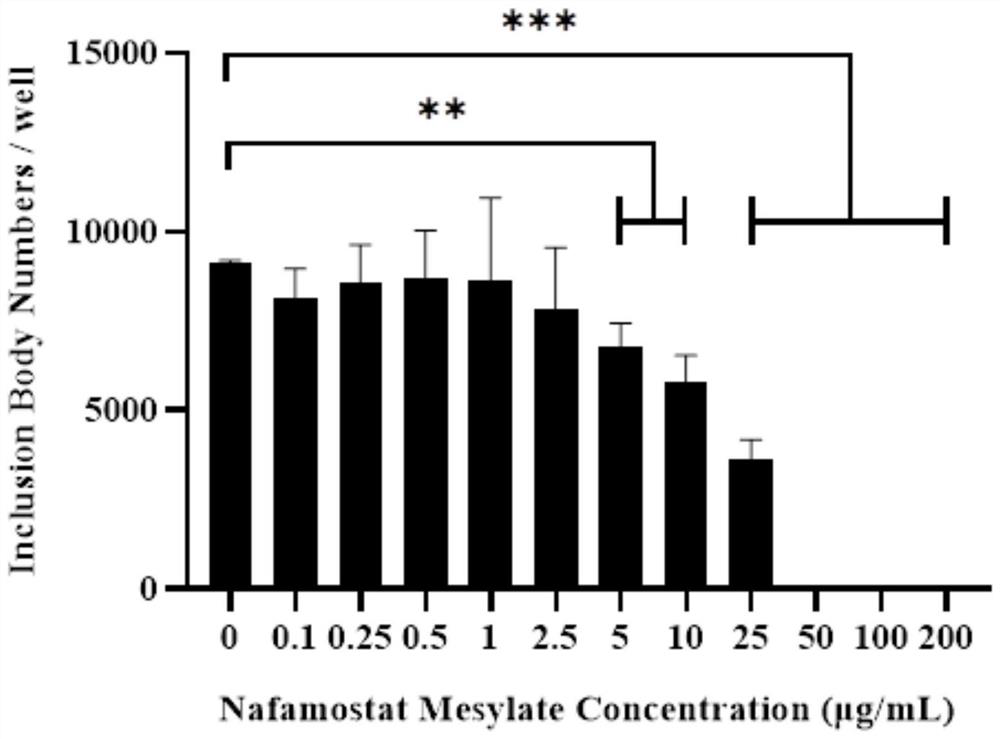
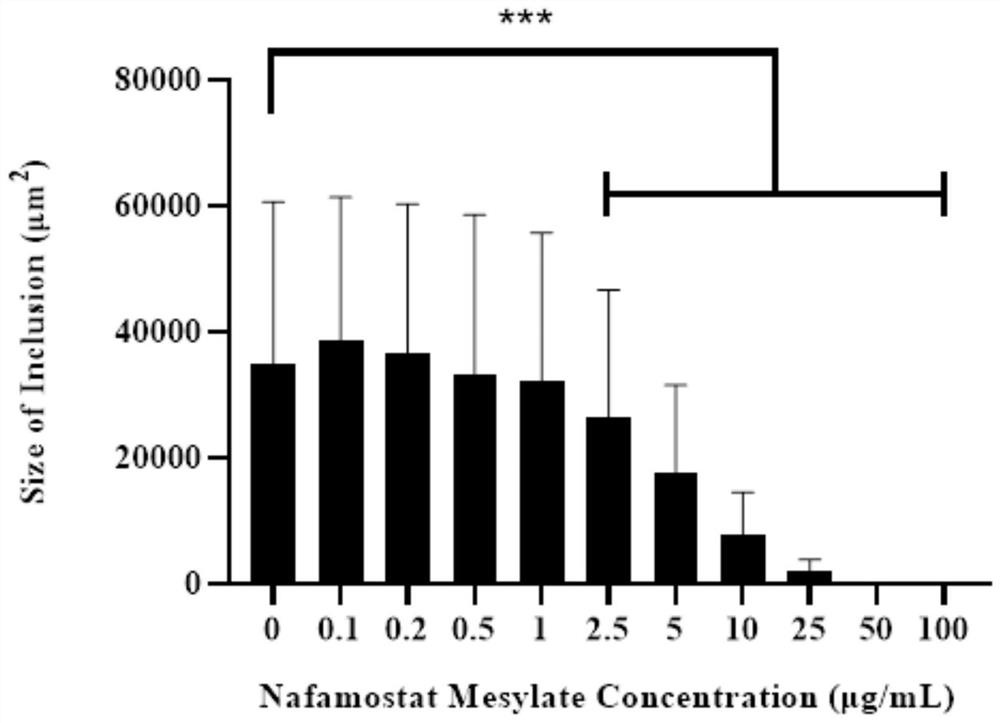
[0045] Inhibitory effect of nafamostat mesylate on genital tract epithelial cells infected by chlamydia
[0046] 1. Experimental materials
[0047] Strains and cells: Chlamydia muridarum (Cm) Cm-mCherry fluorescent strain, constructed by our laboratory, routinely passaged, purified, and preserved. The fluorescent strain introduced the red fluorescent protein mCherry gene into the plasmid of wild-type Cm. The strain comes with Fluorescence, without fixation and antibody incubation, can be used for fluorescence observation in vivo after infection of cells. HeLa229 cells were purchased from the American Culture Collection (ATCC), and were routinely passaged and preserved by our laboratory;
[0048] Main reagents: nafamostat mesylate was purchased from Abcam Company of the United States; high-glucose DMEM medium and trypsin were purchased from Hyclone Company of the United States; fetal bovine serum was purchased from Gibco Company of the United States; cycloheximide, gentamicin,...
Embodiment 2
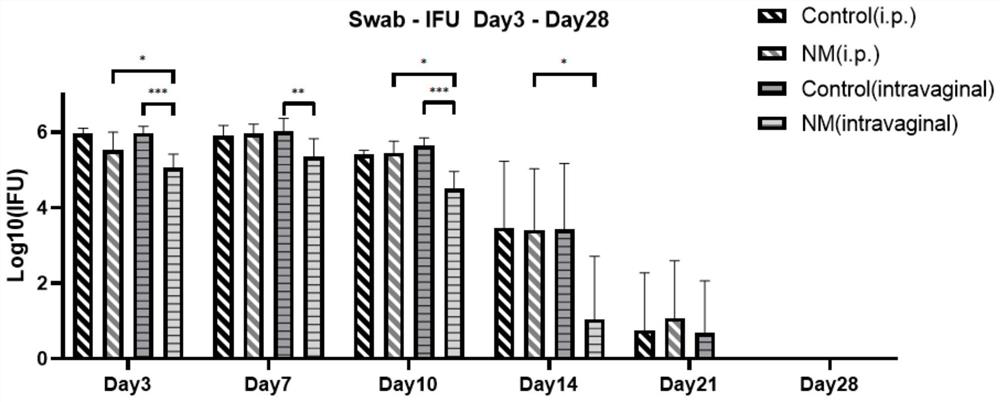
[0056] Effects of Nafamostat Mesylate on the Reproductive Tract of Mice Infected with Chlamydia
[0057] 1. Experimental materials
[0058] Animal: C57BL / 6 mouse, SPF grade, 6 weeks old, female, purchased from Hunan Slake Jingda Experimental Animal Co., Ltd., license number: SCXK (Xiang) 2019-0004;
[0059] Cells and main reagents are the same as in Example 1.
[0060] 2. Experimental method
[0061] Grouping of animals: 5 animals in the abdominal control group, 5 animals in the vaginal control group, 6 animals in the intraperitoneal administration group, and 7 animals in the vaginal administration group.
[0062] Infection model establishment: 5 days before infection with Chlamydia (-5d), each mouse was pre-injected with 2.5 mg of medroxyprogesterone subcutaneously to synchronize the estrous cycle of the mice and facilitate Chlamydia infection. On the day of infection (0d), each mouse was inoculated vaginally with 5×10 5 Chlamydia inclusion body forming units (Inclusion f...
Embodiment 3
[0070] Effects of intravaginal infusion of nafamostat mesylate on cytokines in the upper and lower reproductive tracts of mice infected with chlamydia
[0071] 1. Experimental materials
[0072] Animals, cells, and main reagents are the same as in Example 2.
[0073] 2. Experimental method
[0074] Grouping of animals: 10 vaginal control groups and 10 vaginal administration groups;
[0075] The establishment of the infection model and the way of administration are the same as in Example 2.
[0076] Determination of cytokines in the upper and lower reproductive tracts: 5 mice in each group were randomly killed on the 7th and 14th day after infection, and the upper and lower reproductive tracts were carefully separated. Add upper and lower reproductive tract tissues and 500 μl SPG buffer respectively to the homogenizer, grind on ice, collect the homogenate, centrifuge at 4° C., 500 g for 5 minutes, and collect the supernatant. Use BD TM Cytometric Bead Array kit (BD CBA mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com