Compounds and methods for treatment and diagnosis of chlamydial infection
A technology of chlamydia and antigen, applied in chemical instruments and methods, botany equipment and methods, biochemical equipment and methods, etc., can solve problems such as infertility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0141] EXAMPLES Example 1: Cloning of DNA sequences encoding Chlamydia antigens
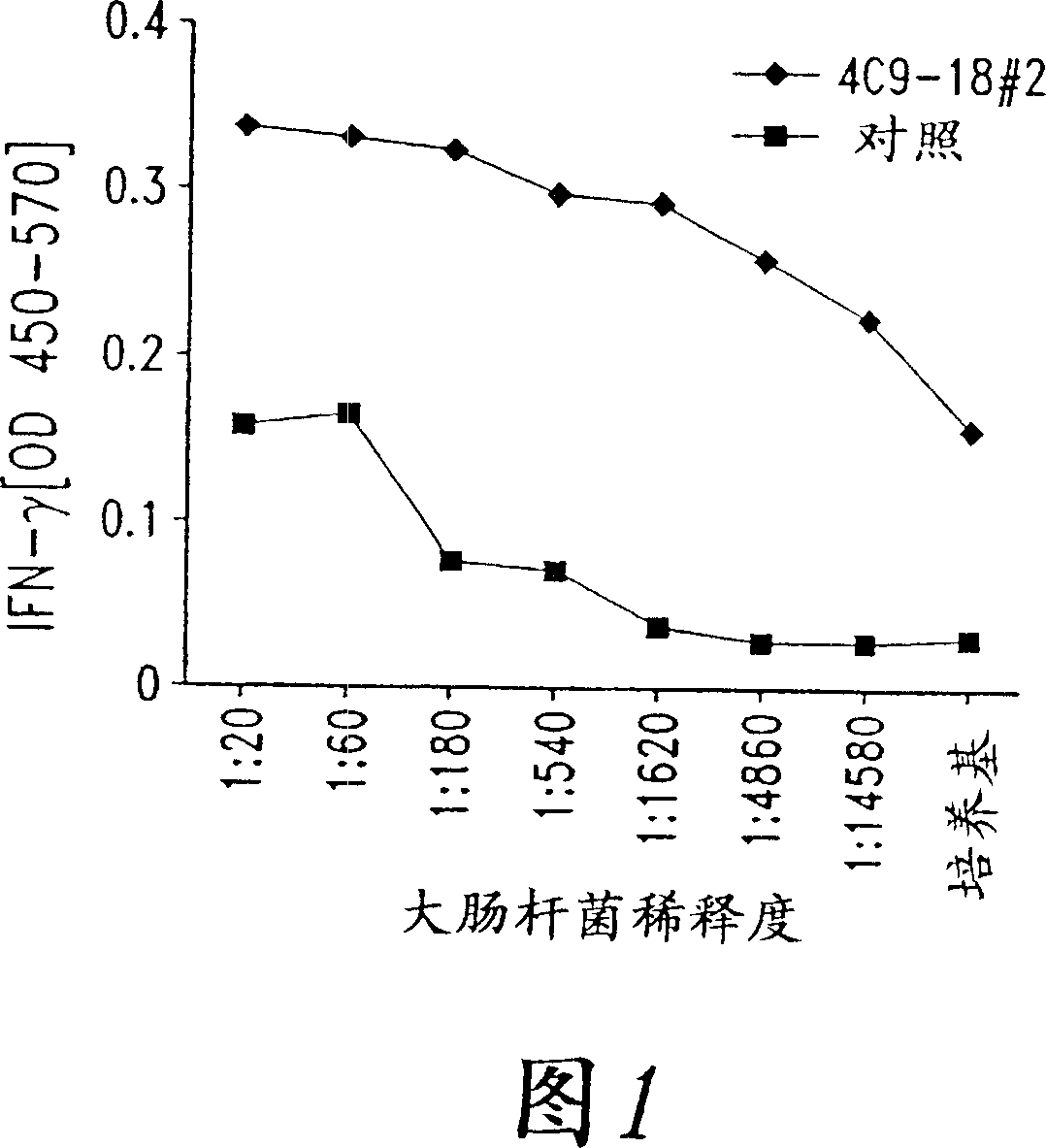
[0142] The Chlamydia antigens of the present invention are isolated by expression cloning of a Chlamydia trachomatis LGV II genomic DNA library substantially as described in Sanderson et al., J. Exp. Med., 182:1751-1757, 1995, which demonstrates immunoreactivity. Induction of PBMC proliferation and IFN-γ production in T cell lines.
[0143] Chlamydia-specific T cell lines were generated by stimulating PBMCs from normal individuals without a history of Chlamydia genital tract infection with primary bodies of Chlamydia trachomatis LGV II. This T cell line, called TCL-8, was found to recognize monocyte-derived dendritic cells infected with C. trachomatis and C. pneumoniae.
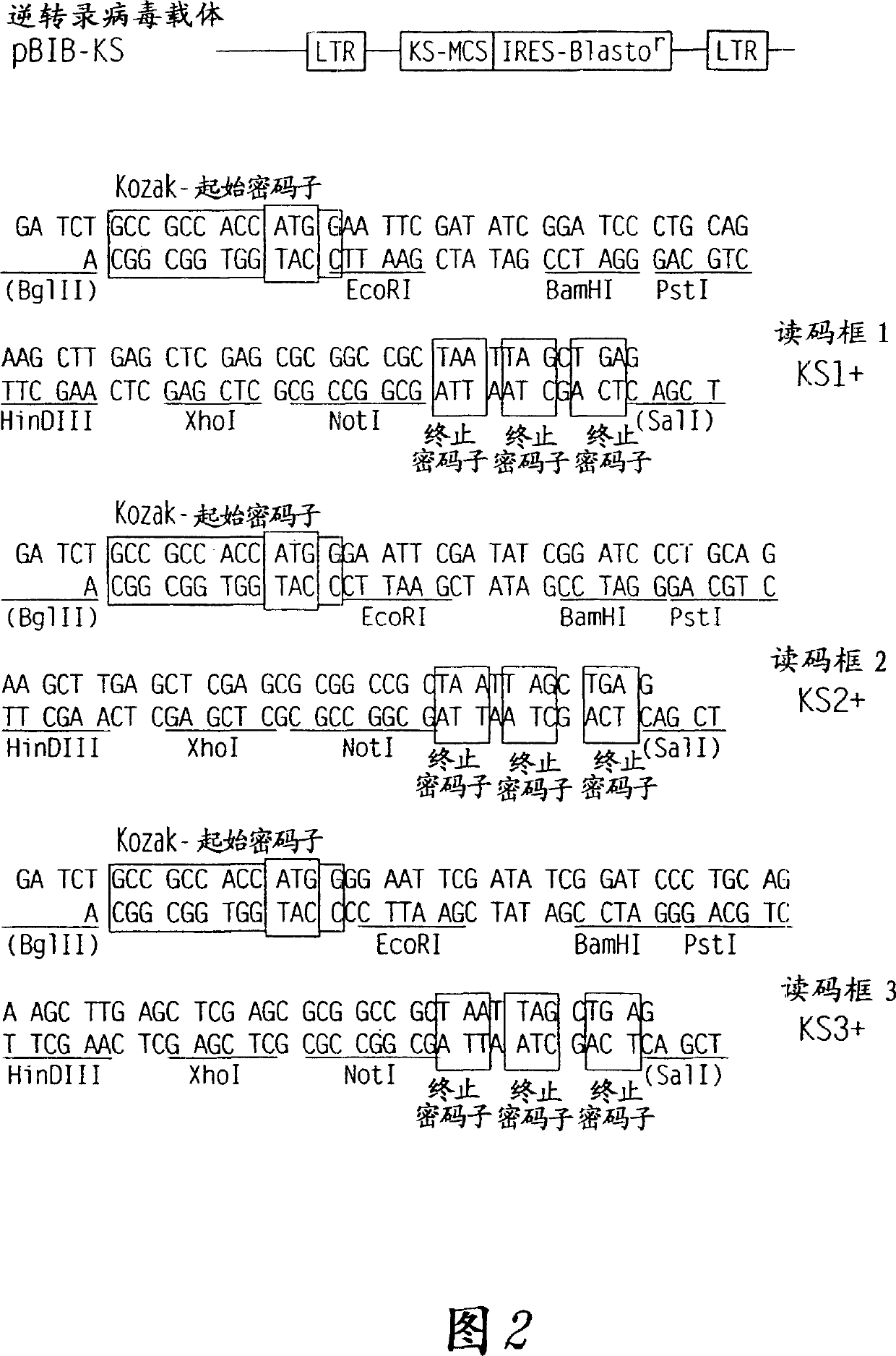
[0144] A randomly sheared genomic library of Chlamydia trachomatis LGV II was constructed in λZAP (Stratagene, La Jolla, CA), and the amplified library was plated in 96-well microtiter plates at a densit...
Embodiment 2
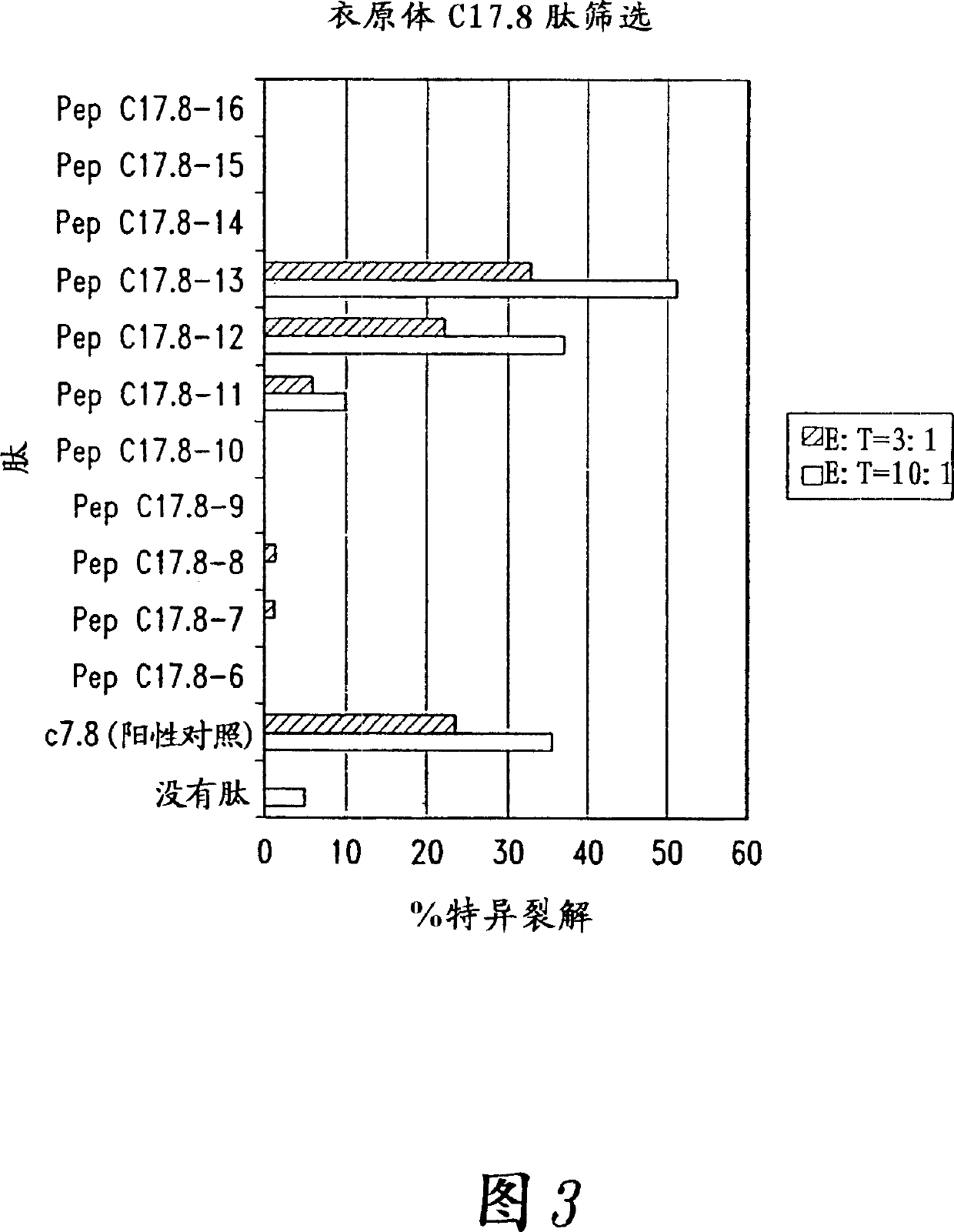
[0157] Additional Chlamydia trachomatis antigens were identified by serological expression cloning. As mentioned above, these studies used pooled sera from several Chlamydia-infected individuals, but used IgA and IgM antibodies as secondary antibodies in addition to IgG. Clones selected by this method have enhanced detection of antigens recognized by the early immune response to Chlamydia infection, the mucosal humoral immune response. The following immunoreactive clones were identified and the inserts containing Chlamydia genes were sequenced: CTL2gam-1 (SEQ ID NO: 290), CTL2gam-2 (SEQ ID NO: 289), CTL2gam-5 (SEQ ID NO: 288), CTL2gam-6-3' (SEQ ID NO: 287, the second genome sequence determined, representing the 3' end), CTL2gam-6-5' (SEQ ID NO: 286, the first genome sequence determined, representing the 5 ' end), CTL2gam-8 (SEQ ID NO: 285), CTL2gam-10 (SEQ ID NO: 284), CTL2gam-13 (SEQ ID NO: 283), CTL2gam-15-3' (SEQ ID NO: 282, The second determined genome sequence represent...
Embodiment 3
[0166] Peptides can be synthesized using HPTU (O-benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate) activated FMOC chemistry on a Millipore 9050 peptide synthesizer. A Gly-Cys-Gly sequence can be attached to the amino terminus of the peptide to provide a means of conjugating or labeling the peptide. Peptides can be cleaved from the solid support using the following cleavage mixture: trifluoroacetic acid:ethanedithiol:thioanisole:water:phenol (40:1:2:2:3). After 2 hours of cleavage, the peptide can be precipitated in cold methyl tert-butyl ether. The peptide pellet was then dissolved in water containing 0.1% trifluoroacetic acid (TFA) and lyophilized prior to purification by C18 reverse phase HPLC. Peptides can be eluted using a gradient of 0-60% acetonitrile (with 0.1% TFA) in water (with 0.1% TFA). After lyophilization of the pure fractions, the peptides can be identified using electrospray mass spectrometry and amino acid analysis. Example 4: Isolation and Ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com