Immunization against chlamydia infection
A chlamydia, immunogenic technology for use in immunology to address issues such as the increase in antibiotic-resistant microbes, where chemotherapy or antibiotic treatment is not a viable long-term strategy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] Standard molecular biology techniques for preparing and purifying polynucleotides are used in the preparation of polynucleotide therapeutics of the invention. For use as a vaccine, the polynucleotides of the invention are formulated according to the various methods outlined below.
[0095] One method utilizes naked polynucleotides without any delivery vehicle. The polynucleotide is simply diluted in a physiologically acceptable solution, such as sterile saline or sterile buffered saline, with or without carrier. When present, the carrier is preferably isotonic, hypotonic or slightly hypertonic, and has a relatively low ionic strength, such as that provided by a sucrose solution, eg, a solution containing 20% sucrose.
[0096] Alternative methods utilize polynucleotides in combination with agents that assist in cellular uptake. Examples of such agents are (i) chemical agents that modify cell permeability, such as bupivacaine (see, e.g., WO 94 / 16737), (ii) liposomes f...
Embodiment 1
[0148] This example illustrates the preparation of plasmid vectors for immunization.
[0149] Chlamydia trachomatis mouse pneumonia (MoPn) isolates were grown in Hela229 cells in Eagle MEM containing 10% fetal bovine serum and 2 mM L-glutamine. MoPn EBs were harvested and purified by a gradient density centrifugation step at 43,000 g for 60 min at 4°C. Purified EBs were washed twice with PBS, centrifuged at 30,000g for 30 minutes, resuspended in sucrose-phosphate-glutamate (SPG) buffer and frozen at -70°C until use.
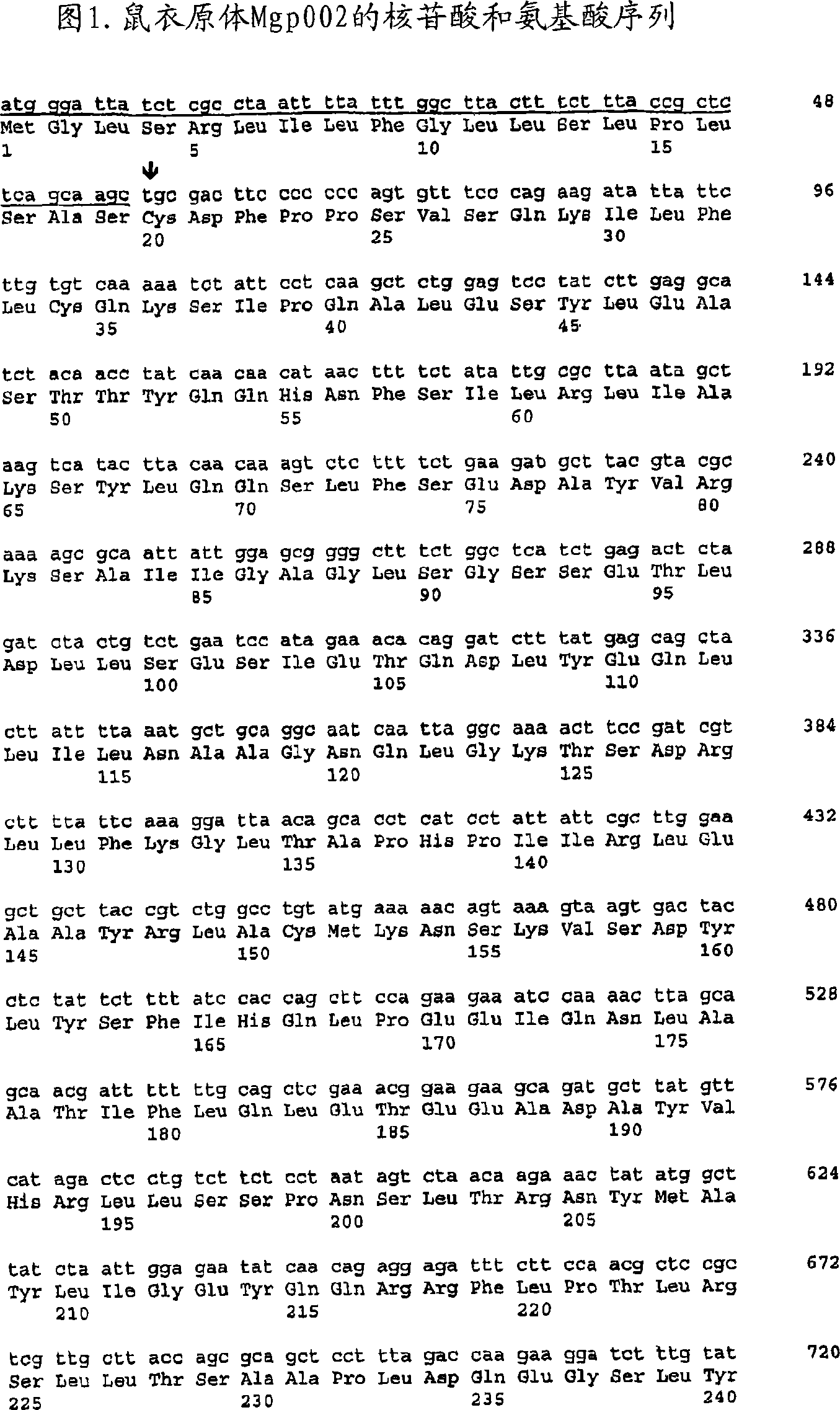
[0150] The nucleic acid molecule encoding the Mgp002 gene is cloned in-frame into the eukaryotic expression plasmid pCAMycHis, and the Myc-His tag exists in the vector. This vector was constructed from pcDNA3.1(-)Myc-His C (Invitrogen, San Diego) and plasmid VR1012 (Vical). Details of the construction are disclosed in PCT Publication WO 00 / 55326, published September 21,2000. Briefly, plasmid pcDNA3.1(-)Myc-His C (Invitrogen) was digested with Spe I and Bam HI ...
Embodiment 2
[0155] This example shows the results of immunization studies using nucleic acid vectors.
[0156] To investigate whether the immune response elicited by nucleic acid immunization was functionally effective, in vivo protective efficacy was assessed according to previously described methods (ref. 20). Briefly, female Balb / c mice (4 to 5 weeks old) were purchased from Charles River Canada (St. Constant, Canada). Mice were immunized intramuscularly and intranasally with plasmid DNA prepared according to the method described in Example 1, see FIG. 3 at three times of 0, 2 and 4 weeks. For each immunization, 200 μl of a total of 200 μg DNA was injected into both quadriceps using a 27 gauge needle (100 μg DNA per injection site). At the same time, 50 μl of 50 μg DNA was delivered to the nostrils of the mice using a micropipette. The droplets were then inhaled by the mice.
[0157] Fourteen days after the last immunization, 2 × 10 3 IFU of Chlamydia trachomatis MoPn EB challenged...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com