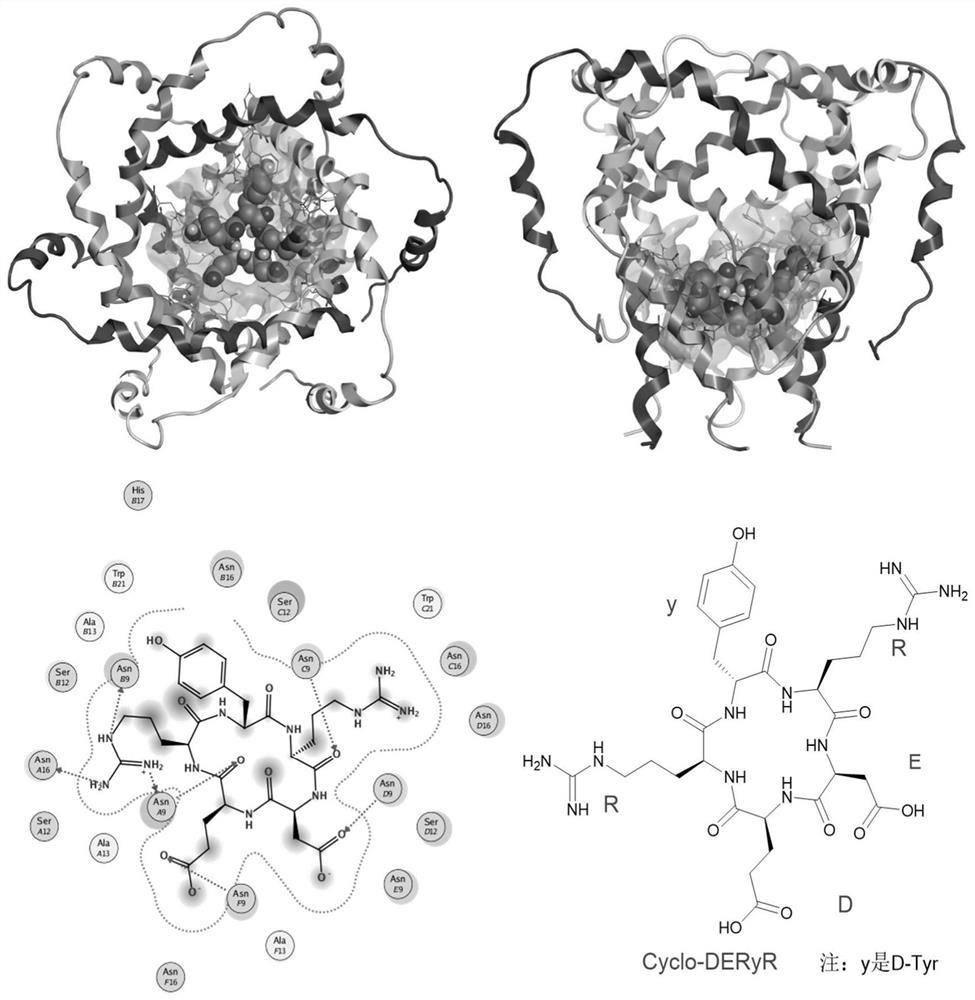
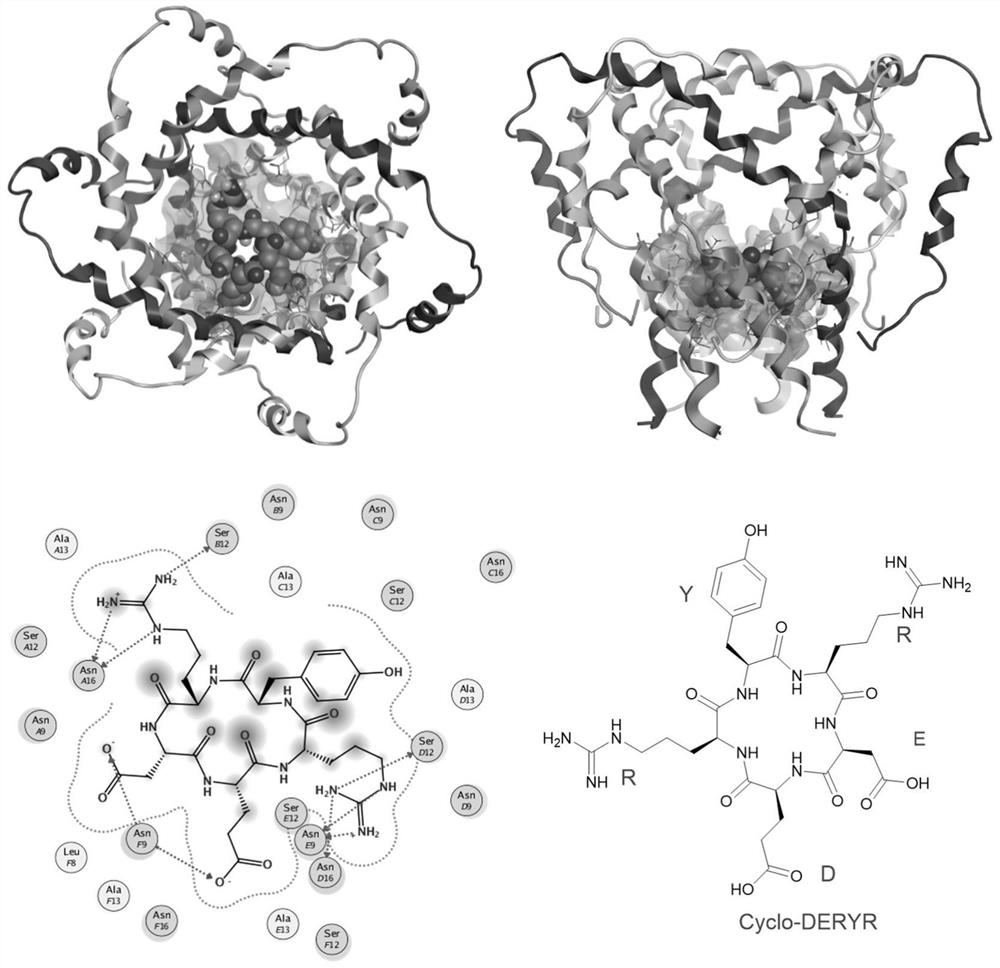
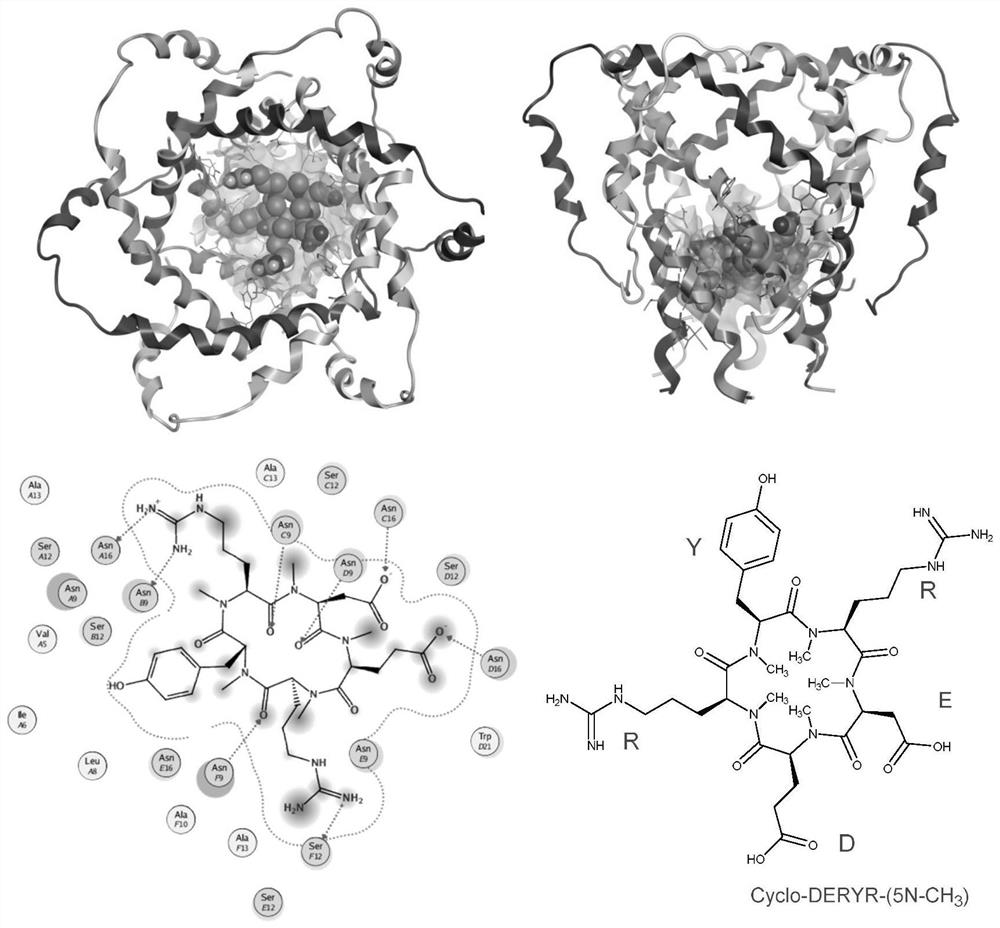
Synthesis method of cyclic pentapeptide and application of cyclic pentapeptide in anti-hepatitis C medicine
A technology of cyclic pentapeptides and uses, which is applied in the field of cyclic pentapeptides and its application in anti-hepatitis C drugs, can solve the problems of disappearance, weakened antiviral efficacy, and difficulty in forming cyclized structures, etc., and achieve good structural stability, The effect of efficient combination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] In order to better understand the present invention, the present invention will be described in detail below in conjunction with specific drawings.
[0027] 1. Preparation of Cyclic Pentapeptide
[0028] 1.1 Raw materials and reagents:
[0029] (1) Protect amino acids and raw materials
[0030] Fmoc-Arg(Pbf)-OH, Fmoc-D-Tyr(tBu)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Asp(OtBu)-OH, etc.
[0031] (2) Condensation reagent
[0032] HBTU, DIEA,
[0033] (3) Solvent
[0034] DMF, DCM, acetonitrile,
[0035] (4) Resin
[0036] 2-Chlorotrityl chloride resin
[0037] (5) Deprotection reagent
[0038] piperidine
[0040] Trifluoroethanol, DCM, TFA, TIS, EDT, H 2 o
[0041] (7) Nitrogen
[0043] (9) Precision electronic balance
[0044] 1.2 Synthesis process:
[0045] (1) Resin swelling
[0046] Put 2-Chlorotrityl Chloride Resin resin into the reaction tube, add DCM (15ml / g), and shake for 30min.
[0047] (2) Take the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com