Application of 6,8-xanthine compound in preparing anti-HBV drugs
A technology of hydroxypurine and hepatitis B virus, which is applied in 6 fields, can solve the problems of many side effects, poor tolerance, high cost, etc., and achieve the effect of inhibiting core protein assembly, good antiviral effect, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
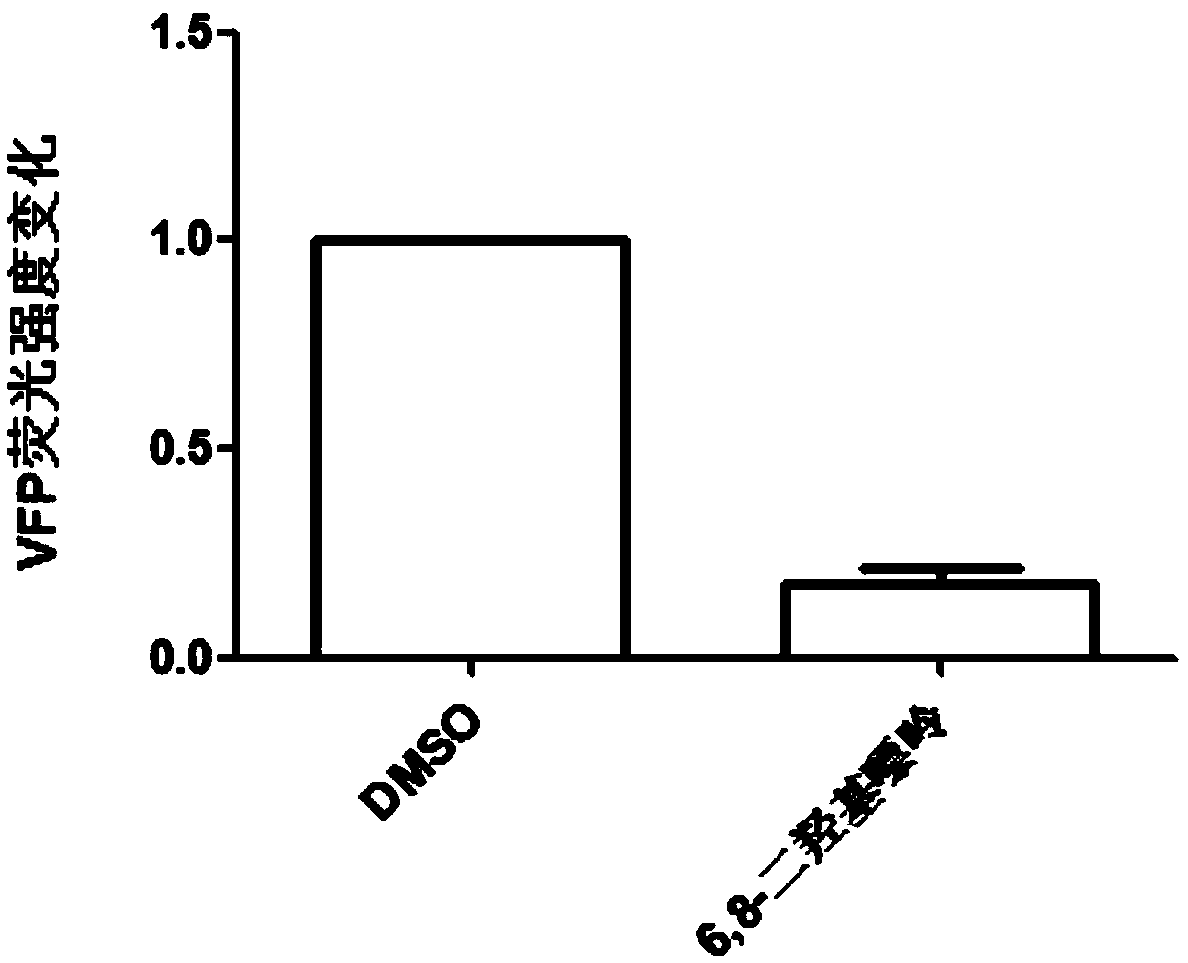
[0027] Study on the Effect of 6,8-Dihydroxypurine on Inhibiting the Reciprocal Binding of HBV c Antigen
[0028] Experimental method: Take well-grown human adrenal cell line 239t cells and inoculate them in a 96-well transparent flat-bottomed plate, with 5×104 cells per well. The medium used is complete medium: high-glucose DMEM, 10% fetal bovine serum and 1% double antibody, the culture condition is 5% carbon dioxide, 37°C; C and pcDNA3.1-HBVcAg-VFP-N two plasmids. For transfection, liposome-encapsulated transfection was used, lipo2000 was used as the reagent, and 20 μl of transfection solution was used. After 4 hours of transfection, compound A to be screened was added, 2 μl per well, with a final concentration of 50 μM. After culturing for 48 hours, the expression of green fluorescent protein VFP was detected. If there is a decrease in the expression of green fluorescent protein VFP, the compound may become an antiviral drug candidate. Experimental results such as figu...
Embodiment 2
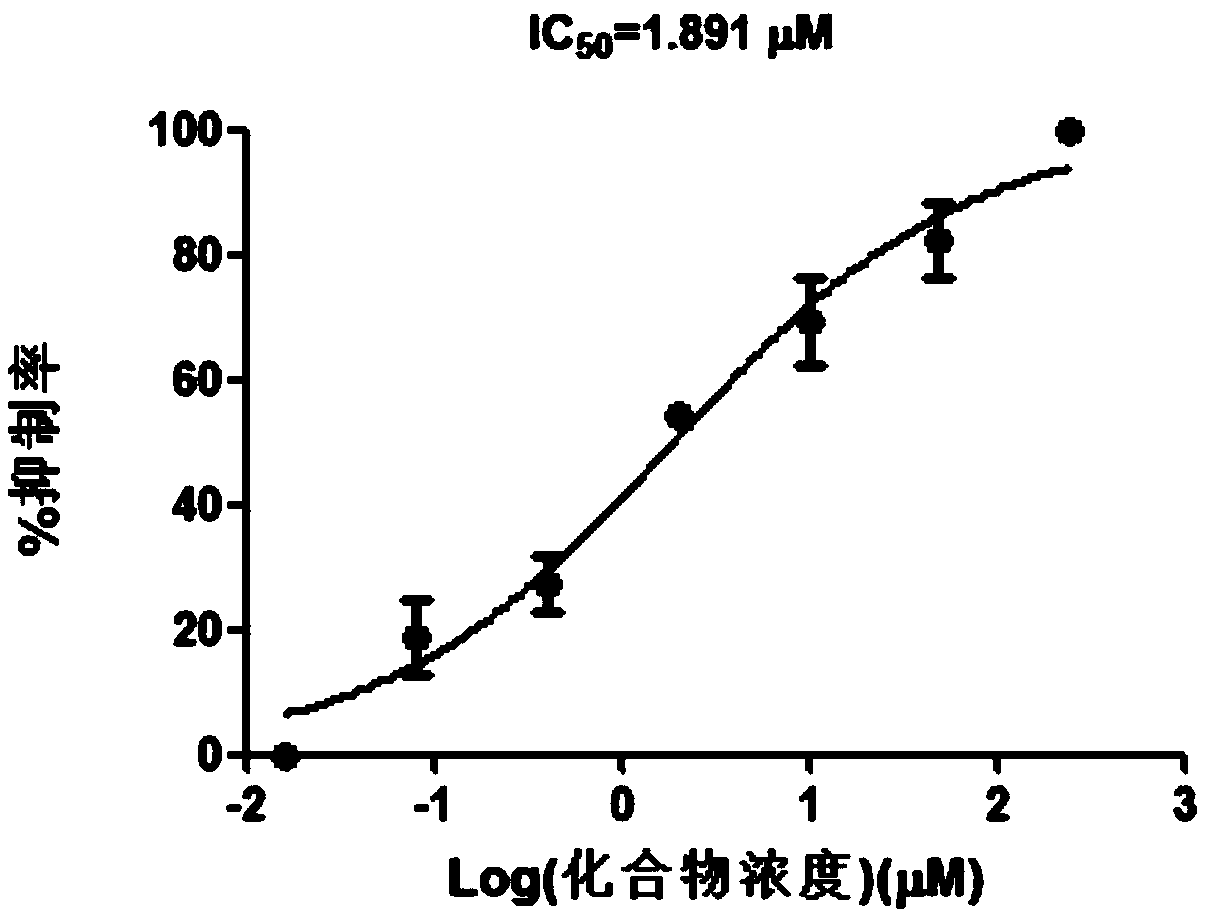
[0030] 6,8-Dihydroxypurine inhibits viral replication of wild-type HBV
[0031] Experimental method: Take the well-grown cell line HepG2.2.15 that can produce wild-type HBV virus, the amount of cells is 2×104 / well, add compound A after 24 hours of laying on a 96-well plate, and compound 2 μl per hole (final concentration 250 μM, 50 μM , 10 μM, 2 μM, 0.4 μM, 0.08 μM, 0.016 μM, 0 μM); use 2% Triton X-100 to treat the collected supernatant, harvest the cultured cell supernatant for 8 days, and then detect the HBV content in the cell culture supernatant DNA content. Experimental results such as figure 2 shown. It can be seen from the experimental results that 6,8-dihydroxypurine has a good effect of inhibiting the replication of wild-type HBV virus, and its IC50 is 1.891 μM.
Embodiment 3
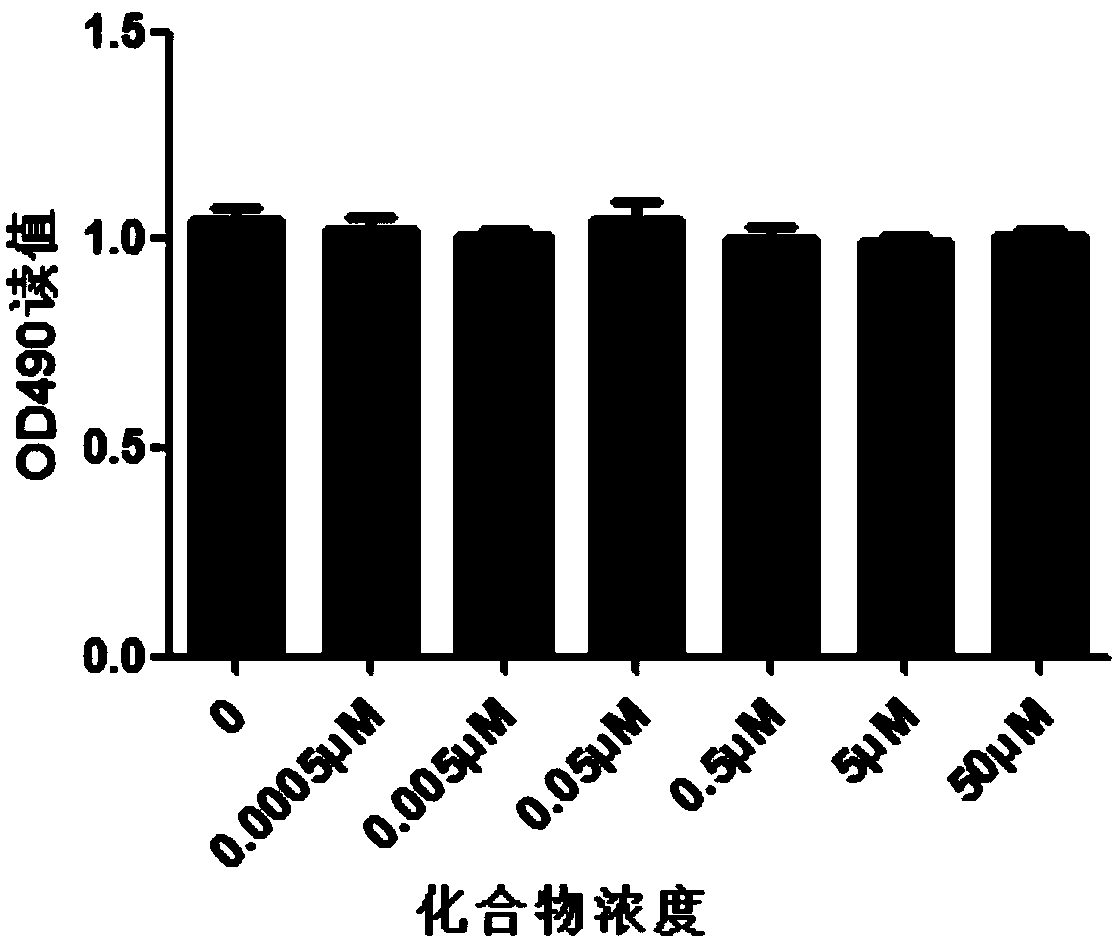
[0033] Cytotoxicity of 6,8-dihydroxypurine in 293t cells
[0034] Experimental method: use DMEM culture medium containing 10% fetal calf serum to prepare 293t into a single cell suspension, inoculate 1000 cells per well into a 96-well plate with a volume of 200ul per well; 2 μl, the final concentration is 50 μM, 5 μM, 0.5 μM, 0.05 μM, 0.005 μM, 0.0005 μM, 0 μM; after 48 hours of culture, add 20 μl of MTS solution to each well, and continue to incubate in the incubator for 2 to 4 hours; The light absorption value of each well was measured on an enzyme-linked immunosorbent monitor, and the cytotoxicity of the compound to 293t cells was observed. Experimental results such as image 3 shown. It can be seen from the experimental results that 6,8-dihydroxypurine has low toxicity, and it shows no cytotoxicity in 293t cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com