Antibody of humanized anti-anthrax protective antigen PA and application thereof
A protective antigen, anti-anthrax technology, applied in the application of preventive medicine, preparation of anthrax treatment field, to achieve high affinity, high protection, high specificity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation and Screening of Murine Monoclonal Antibody PA6
[0038] 1) Prepare anti-PA monoclonal antibody using hybridoma technology, the specific steps are as follows:
[0039] 1. Preparation of antigen (PA83 protein)
[0040] Construct the PA83 expression plasmid.
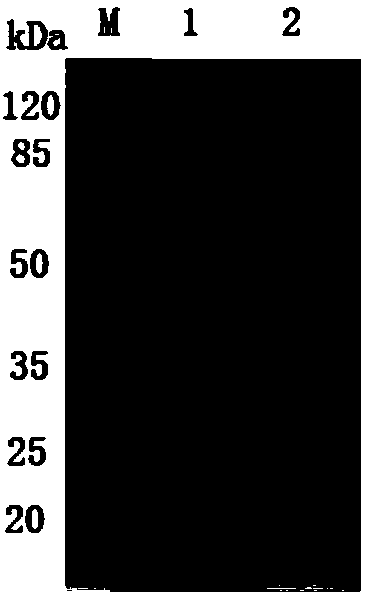
[0041] The above plasmid was transformed into Escherichia coli BL-21 competent cells, positive transformants were screened for prokaryotic expression, and the expression product was detected by SDS-PAGE, and a recombinant protein of 83kd was obtained, which was consistent with the molecular weight of PA83 protein. The target protein was purified with an analysis column (Amersham Company, USA) to obtain the PA83 recombinant protein with His tag, which was named PA83 protein. See figure 1 .
[0042] 2. Preparation of PA-resistant hybridoma cell lines (all mouse myeloma SP2 / 0 cell lines for hybridoma technology are derived from pure-line BALB / c mice):
[0043] The mice were immunized by subcu...
Embodiment 2
[0061] The preparation of embodiment 2hmPA6 antibody
[0062] After the detection of the mouse monoclonal antibody above, we selected the PA6 hybridoma cell line to prepare the human-mouse chimeric antibody hmPA6.
[0063] 1) Amplification and verification of antibody variable region gene fragments:
[0064] The PA6 antibody hybridoma cells were cultured to the logarithmic growth phase, and the total RNA was extracted by the Trizol-chloroform-isopropanol method; the dried total RNA was dissolved in 20 μL of water, and the OD260 / OD280 was measured, and the value was 1.9. Take 14 μL of RNA for reverse transcription, use the mRNA in the total RNA as a template, and use OligodT 15 Single-stranded cDNA was obtained by reverse transcription and amplification.
[0065] Design 19 VH upstream primers and 17 Vκ upstream primers, 4 VH downstream primers and 3 Vκ downstream primers Primers:
[0066] Vκ5' upstream primer:
[0067] Vκ-1
[0068] 5’-GGG CCC AGG CGG CCG AGC TCG AYA TCC A...
Embodiment 3
[0204] Functional activity identification of embodiment 3hmPA6 antibody
[0205] 1) ELISA
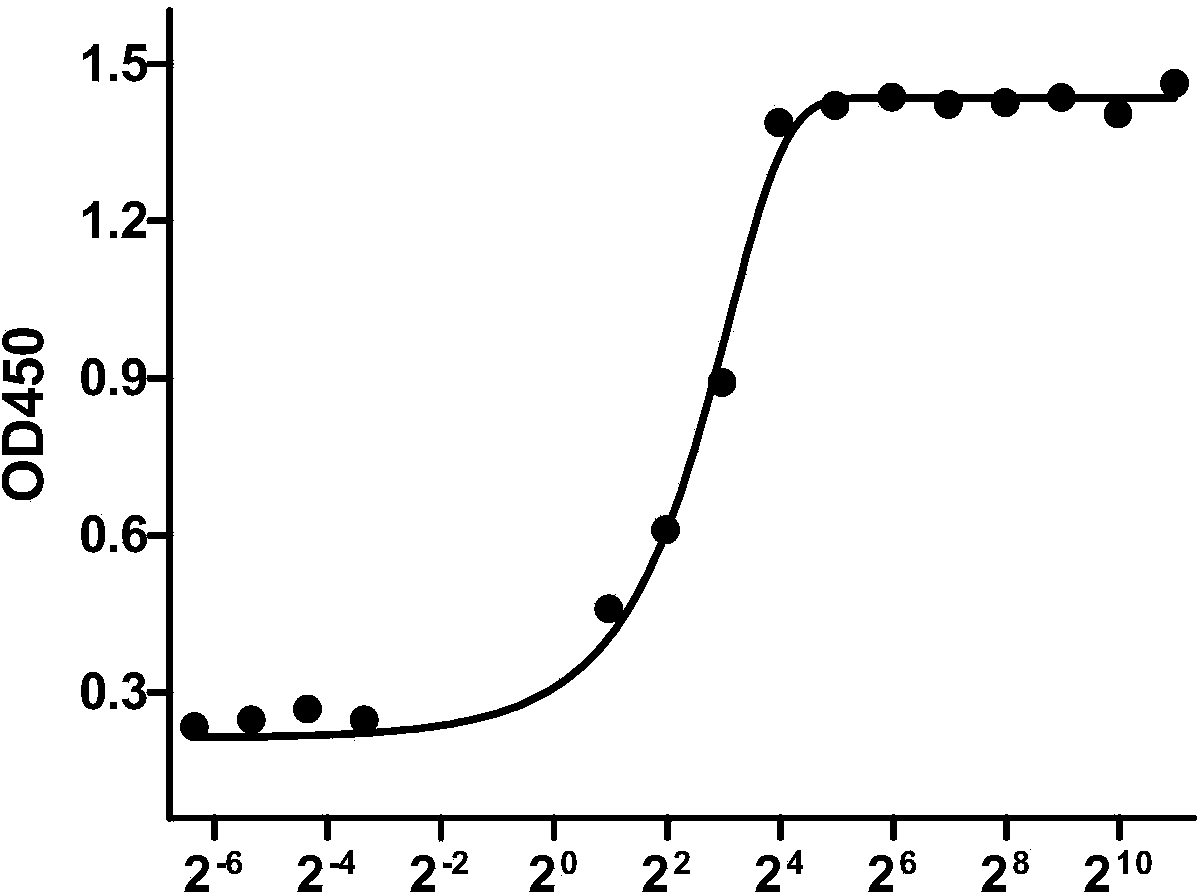
[0206] Dilute the attenuated strain PA83 protein (gifted by the Plague Department of the Chinese Center for Disease Control and Prevention) with coating solution (0.1M carbonate buffer, pH9.6) to 2 μg / mL to coat the ELISA 96-well plate, add 100 μL to each well, 4 °C Overnight; block with PBST (PBS containing 0.5% Tween20) 5% skimmed milk-washing buffer, incubate at 37°C for 2 h; after washing with PBST for 5 times, add 100 μL anti-PA human-mouse chimeric full-molecule IgG (2 μg / mL initial concentration, 14 concentration gradient dilutions) at 37°C for 2h; 100μL / well of goat anti-human secondary antibody diluted 1:4000 was added to the well, and incubated at 37°C for 1h; peroxidase substrate chromogenic solution 100μL / well After 15 minutes at room temperature, stop the reaction with 2M sulfuric acid, and use dual wavelength 450nm / 690nm for colorimetry on the machine.
[0207] The resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com