Temperature stable vaccine formulations
A stable and vaccine technology, applied in the field of freeze-dried and frozen vaccine preparations, can solve problems such as loss of potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1: Freezing / thawing of liquid rPA and AVA vaccines with and without trehalose
[0116] rPA102 vaccine formulations were prepared with and without trehalose as outlined in Table 1 below.
[0117] Table 1. Trehalose Preparations Used for Freeze / Thaw Analysis
[0118]
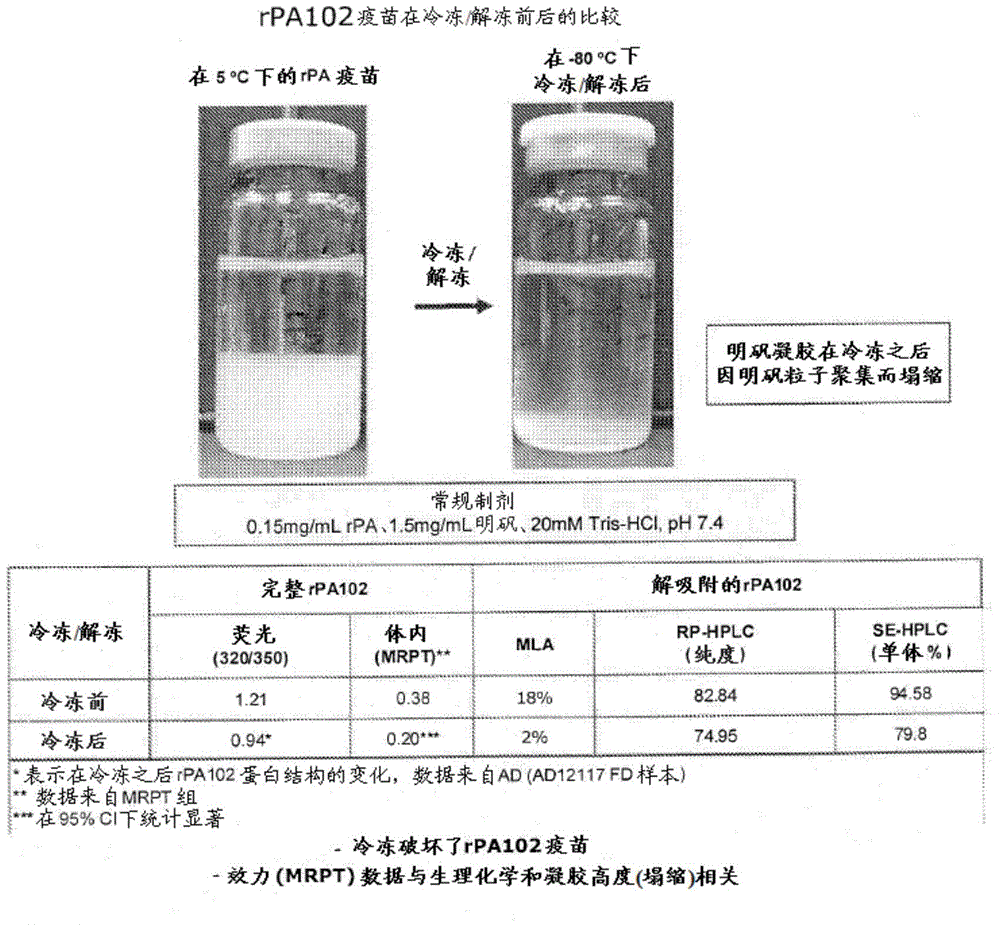
[0119] After compounding, each sample was divided into two 8ml aliquots in 10ml glass tubes. For each sample, one tube was placed at -80°C after gentle mixing overnight and the other tube was placed at 2-8°C after gentle mixing overnight.
[0120] Samples stored overnight at -80°C were thawed on the lab bench for several hours (>3-4 hours) the next day, then observed and compared to samples brought to room temperature at 2-8°C. Photograph the samples and measure the total liquid height and ALHYDROGEL (aluminum hydroxide) height. Comparison of conventional rPA102 vaccine before and after freezing / thawing. figure 1 are photographs comparing rPA Control 1 samples at 2-8°C (labeled ...
Embodiment 2
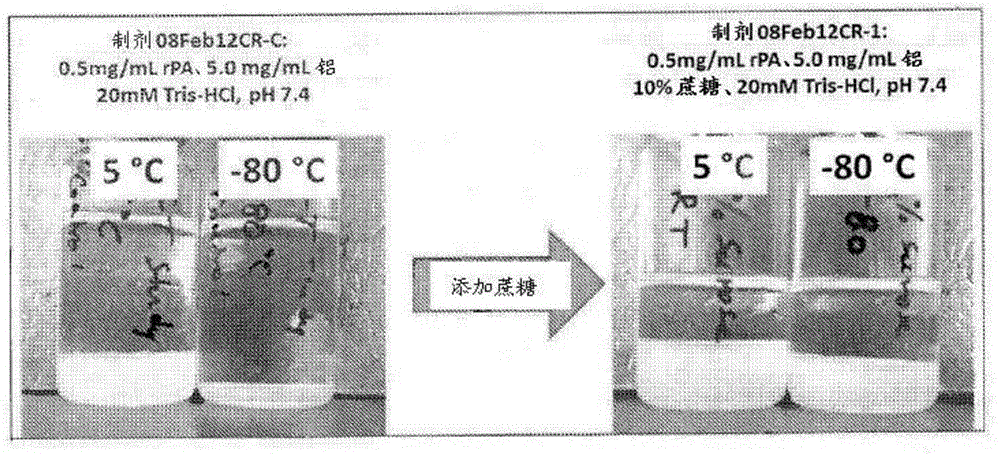
[0125] Example 2: Freezing / thawing of liquid rPA vaccines with and without sucrose
[0126] The rPA102 vaccine formulations were prepared with and without sucrose as outlined in Table 3 below.
[0127] Table 3. Sucrose preparations used for freeze / thaw analysis
[0128]
[0129] After compounding, each sample was divided into two 10ml aliquots in 10ml glass tubes. For each sample, one tube was placed at -80°C after gentle mixing overnight and the other tube was placed at 2-8°C after gentle mixing overnight.
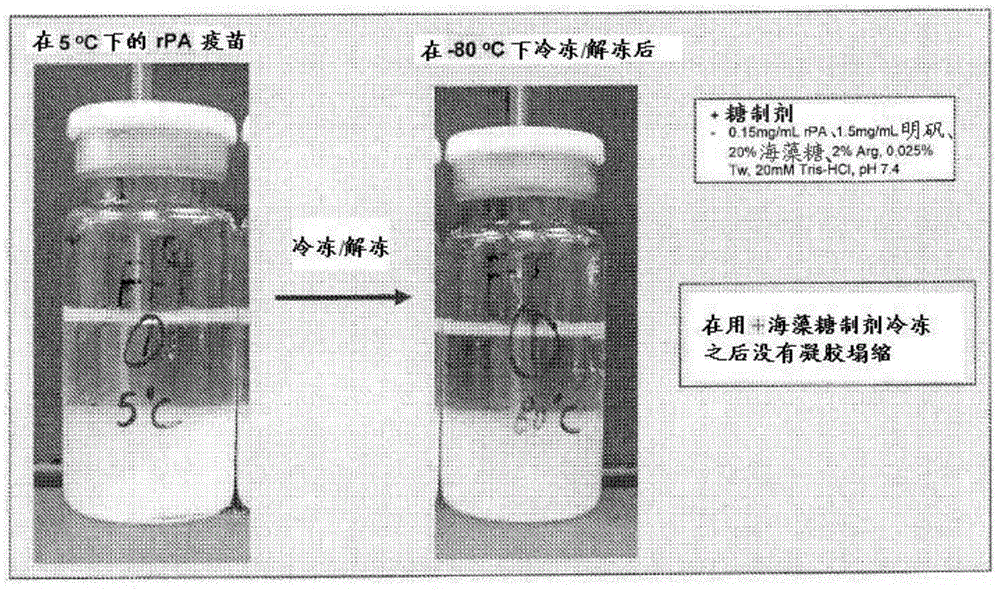
[0130] Samples stored overnight at -80°C were thawed on the laboratory bench for 2-3 hours the next day before observation. image 3 Contains photographs of each formulation from 2-8°C (marked 5°C) and -80°C after both had reached room temperature. As shown, after thawing, gel collapse occurred in both -80°C samples (rPA Control 2 and rPA Test 2) compared to samples still refrigerated at 2-8°C. However, the amount of gel collapse was significantly gre...
Embodiment 3
[0131] Example 3: In Vivo Mouse Potency Analysis
[0132] Lyophilized vaccines were prepared as outlined in Table 4. The dried vaccine was reconstituted with water for injection to a final rPA concentration of 0.15 mg / ml (75 μg / 0.5 ml dose), followed by 10-fold dilution in saline to obtain a dose level of 0.1 (DL).
[0133] Female CD-1 mice, 5-8 weeks old and weighing 20-25 grams, were each used in this study. 0.1 DL vaccine (0.5 ml) was injected intraperitoneally into groups of 20 female CD-1 mice, and serum was collected on day 28 to assess neutralization of anthrax LT cells in mice in a toxin neutralization assay (TNA) The ability to be toxic.
[0134] Table 4. Lyophilized formulations used for mouse potency assays
[0135]
[0136] The immunogenicity of the rPA102 preparation was studied by calculating the neutralization factor (NF50). Neutralization factor NF50 is defined as follows:
[0137]
[0138] wherein the effective dose 50% (ED50) refer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com