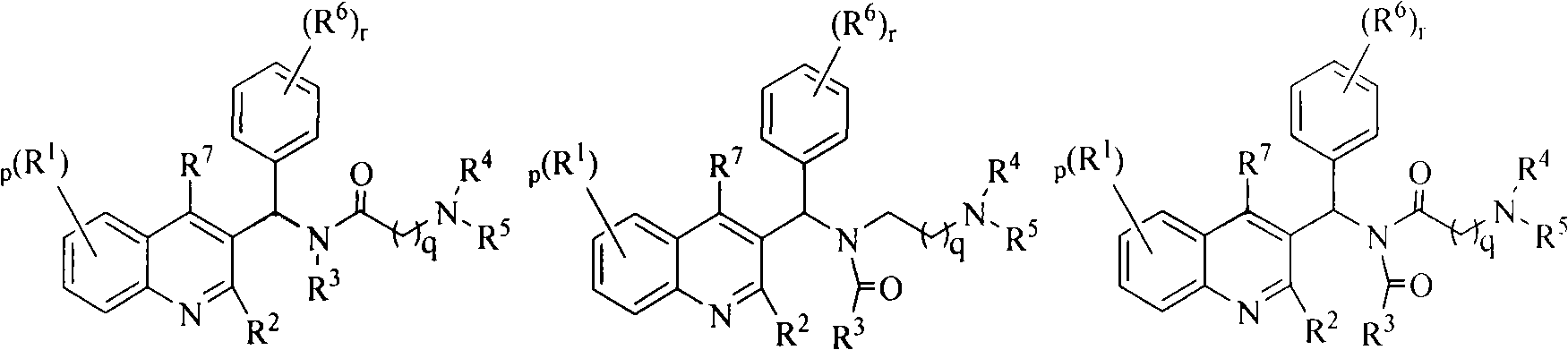
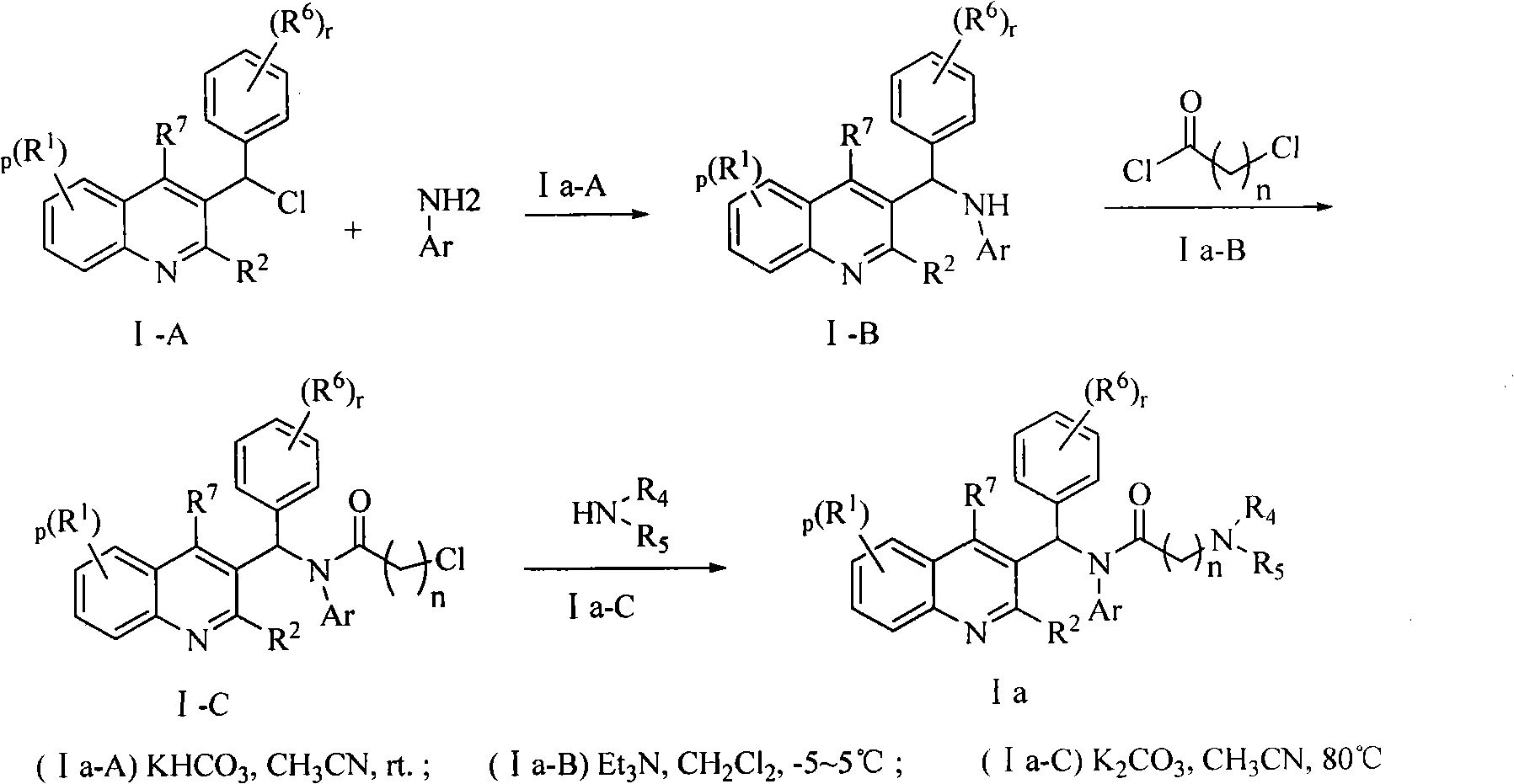
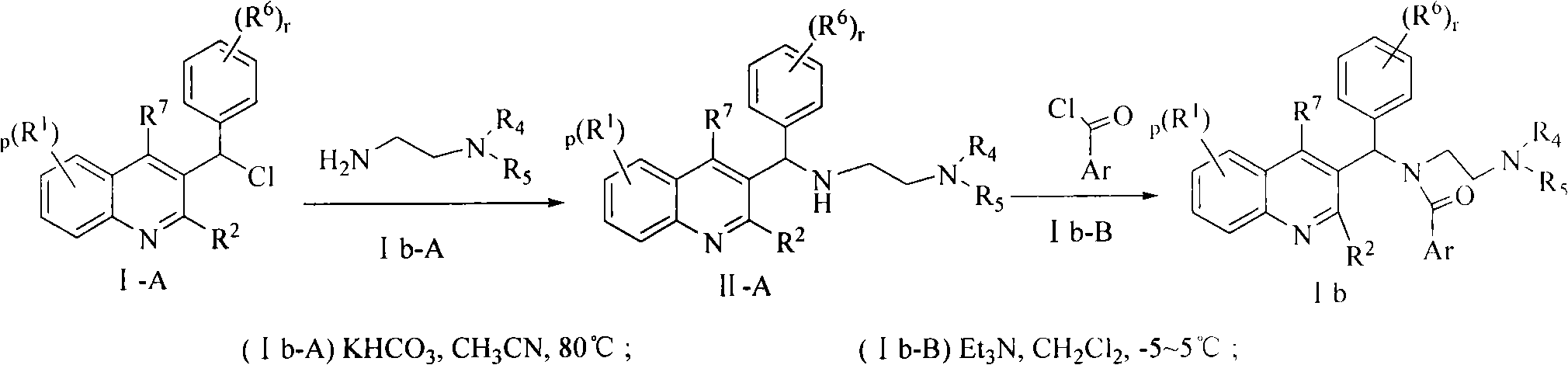
Quinoline derivatives, preparation method and applications thereof
A technology of derivatives and quinolines, applied in the field of medicinal chemistry, can solve problems such as lack of market opportunities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Preparation of representative reaction intermediates:
[0043] Synthetic route I
[0044] 1.1N-(4-bromophenyl)-3-phenylpropanamide (N-(4-bromophenyl)-3-phenylpropanamide, number: M-03)
[0045]
[0046] Reagents and reaction conditions: (a).SOCl 2 , CHCl 3 , reflux; (b).Et 3 N, CH 2 Cl 2 , -5~5℃
[0047] Experimental operation: (a) In a 250ml single-necked bottle, add 20.0g (0.13mol) of 3-phenylpropanoic acid (3-phenylpropanoic acid) and 20ml of dichloromethane, stir to dissolve, add 15ml of thionyl chloride dropwise, Heated to reflux for 3h. After the reaction was completed, the remaining thionyl chloride and the solvent were distilled off to obtain 22.0 g of light yellow liquid with a yield of 98.2%, which was directly carried out to the next reaction. (b) In a 500ml single-necked bottle, add 21.0g (0.12mol) of p-bromoaniline (M-02) and 200ml of dichloromethane, stir to dissolve, dropwise add 22ml of triethylamine, and drop 3 -22.0 g (0.13 mol) of phenylpr...
Embodiment I-01
[0085] N-((6-bromo-2-methoxyquinolinyl-3-yl)(phenyl)methyl)-2-dimethylamino-N-(naphthyl-1-yl)acetamide
[0086] (N-((6-bromo-2-methoxyquinolin-3-yl)(phenyl)methyl)-2-(dimethylamino)-N-(naphthalen-1-yl)acetamide
[0087]
[0088] Reagents and reaction conditions: (CH 3 ) 2 NHHCl, KHCO 3 , CH 3 CN, reflow
[0089] Experimental operation: Add 1.0g (0.002mol) N-((6-bromo-2-methoxyquinolinyl-3-yl)(phenyl)methyl)-2-chloro- N-(Naphthyl-1-yl)acetamide (N-((6-bromo-2-methoxyquinolin-3-yl)(phenyl)methyl)-2-chloro-N-(naphthalen-1-yl)acetamide, Code: M-08), 0.3g (0.003mol) dimethylamine hydrochloride, 1.8g (0.018mol) potassium bicarbonate and 30ml acetonitrile, heated to reflux overnight. Evaporate the solvent to dryness, add water, extract with (20ml×3) dichloromethane, combine the organic phases, dry (MgSO 4 ), filtered, and the solvent was evaporated to dryness to obtain a brown solid crude product. Purified by column chromatography, 0.8 g of the target product was obtained ...
Embodiment I-02
[0091]Example 1-02: 2-(benzyl (methyl)amino)-N-((6-bromo-2-methoxyquinolinyl-3-yl)(phenyl)methyl)-N-( Naphthyl-1-yl)acetamide
[0092] 1 H NMR (300MHz, CDCl 3 )δ2.28 (S, 3H; NCH 3 ), 3.27 (S, 2H; CH 2 ), 3.62 (S, 2H; NCH 2 ), 3.96 (S, 3H; OCH 3 ), 6.14 (S, 1H; CH), 6.65-7.56 (m, 7H; naphthalenering), 7.26-7.13 (m, 10H; benzene ring), 7.68-7.94 (m, 4H; quinoline ring); MS (ESI ( +))m / s 630(M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com