Mycobacterium tuberculosis recombinant fusion protein EECC as well as preparation method and application thereof
A technology for Mycobacterium tuberculosis and fusion protein, which is applied in fusion polypeptide, recombinant DNA technology, chemical instruments and methods, etc., can solve the problems of low specificity, increased diagnostic cost, and high dosage of antigen reagents, and achieves high specificity and reduced The lower limit of detection and the effect of good promotion and application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of Mycobacterium tuberculosis recombinant fusion protein EECC
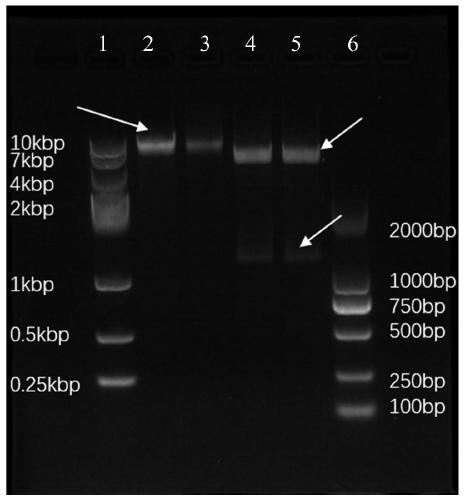
[0054] According to the published genome sequence of Mycobacterium tuberculosis H37Rv (http: / / genolist.pasteur.fr / Tubercu List / ), the amino acid of ESAT6 protein (referred to as E protein) (as shown in SEQ ID NO.5) and the base of the coding gene were obtained base sequence (as shown in SEQ ID NO.6); and the amino acid sequence of CFP10 protein (referred to as C protein) (as shown in SEQ ID NO.7) and the base sequence of the coding gene (as shown in SEQ ID NO.8). The ESAT6 protein and the CFP10 protein are connected in series in the manner of ESAT6-ESAT6-CFP10-CFP10 to obtain the amino acid sequence of the EECC protein (as shown in SEQ ID NO.1) and the base sequence of the coding gene (as shown in SEQ ID NO.3) . The sequence shown in SEQ ID NO.3 was entrusted to Shanghai Bioengineering Co., Ltd. for codon optimization, and an NcoI restriction site (CCATGG) was added to the 5' end of t...
Embodiment 2
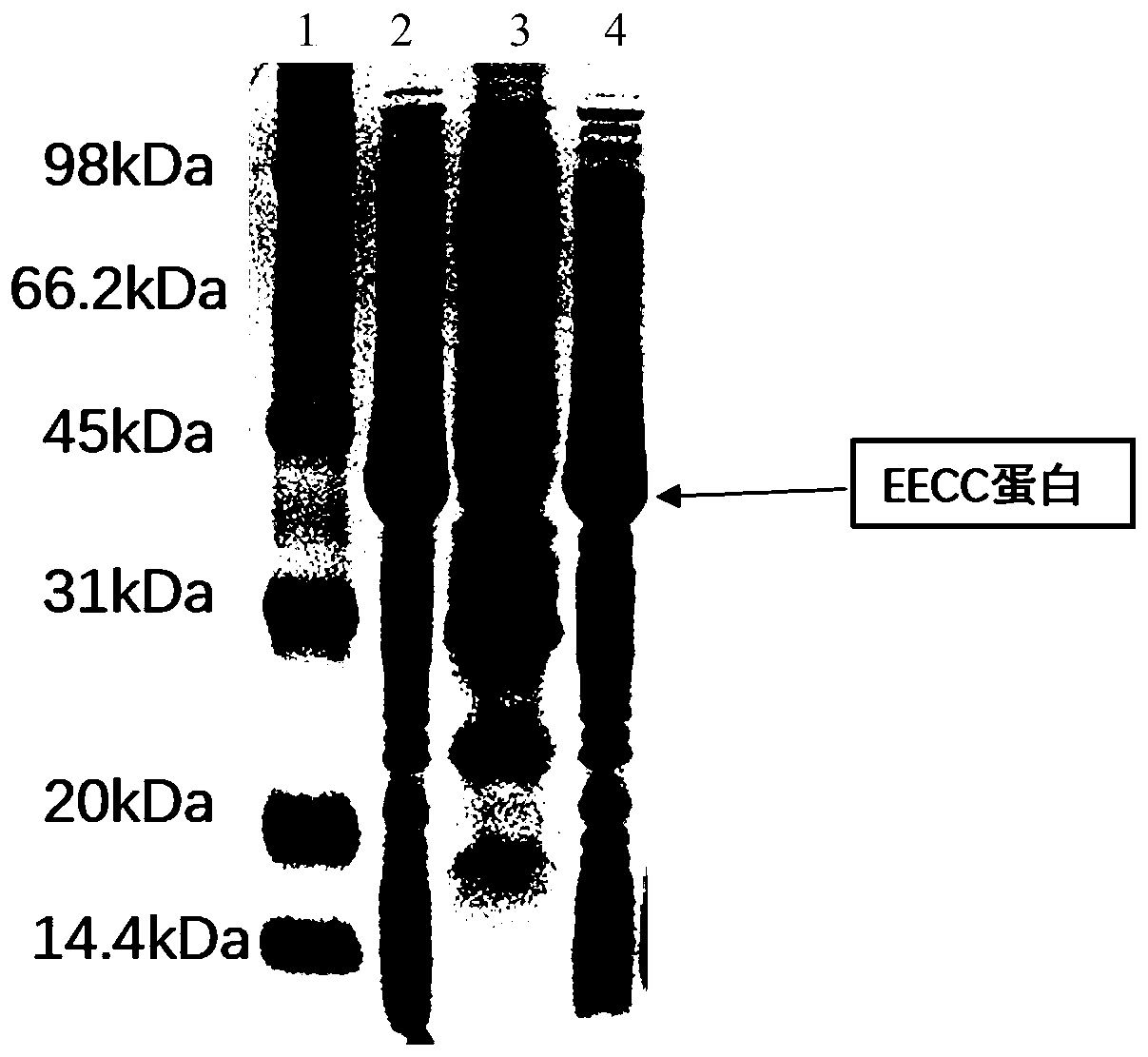
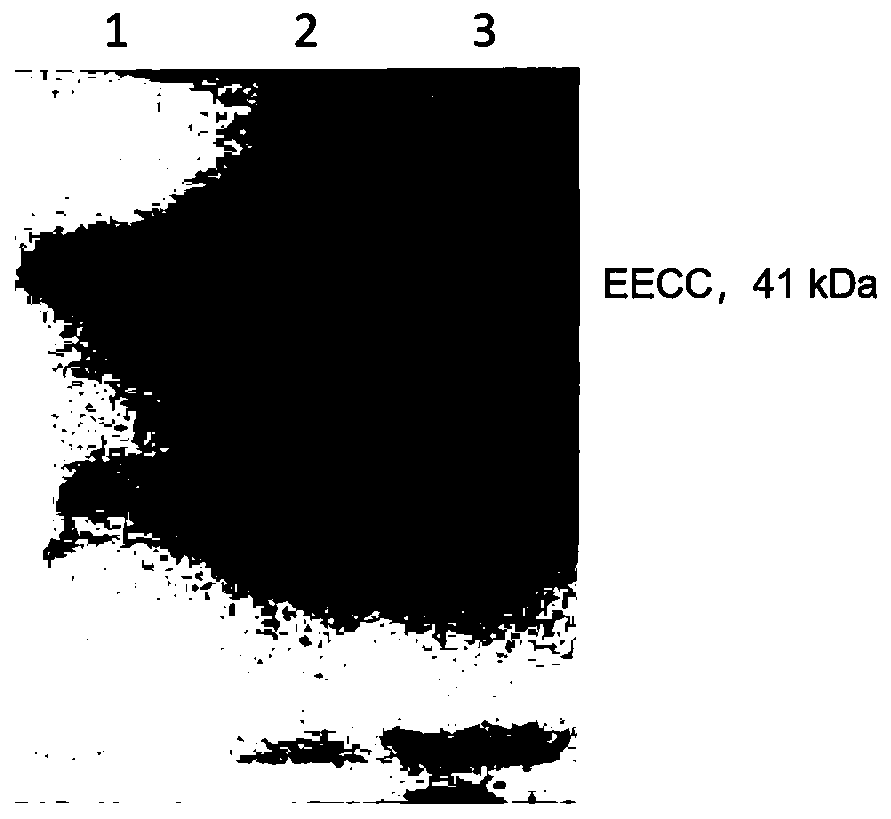
[0056] Example 2 EECC protein extraction, purification and identification
[0057] Cultivate pET28a-EECC / BL21(DE3) engineering bacteria and induce expression of EECC protein. The extraction and purification methods of EECC protein are as follows:
[0058] 1. Bacteria fragmentation
[0059] Pressurize the high-pressure homogenizer gradually to 700±50bar. When the pressure reaches the requirement, put the liquid outlet pipe into the empty beaker, which is the first crushing liquid. The crushing operation was repeated three times, and the cell crushing liquid was collected.
[0060] 2. Dilution and filtration
[0061] Dilute the centrifuged supernatant by 2-3 times the volume with the bacterial cell disruption buffer, and control the pH of the feed solution between 7.1-7.5. Filtration was performed using a 0.45 μm Bursafil filter. Collect the filtrate.
[0062] 3. Anion exchange chromatography (Capto Q)
[0063] The eluted sample was loaded at a flow rate of 300mL / min, and ...
Embodiment 3
[0074] The skin test of embodiment 3 Mycobacterium tuberculosis sensitized guinea pigs
[0075] Healthy SPF grade white guinea pigs without any experiments, weighing 300g-500g. Take out one tube of frozen Mycobacterium tuberculosis, dissolve it naturally at room temperature, and dilute it 10 times with normal saline; inject 0.5ml of the diluted bacteria solution subcutaneously into the groin of each guinea pig’s hind leg, and test the skin of the guinea pig 5-6 weeks after sensitization. The guinea pigs were depilated on both sides of the spine, and 0.2ml of EECC samples of each dilution concentration and TB-PPD and EC standards were injected intradermally. The double-blind method measures the longitudinal diameter and transverse diameter (mm) of redness and / or induration at the injection site respectively at 24 and 48 hours, and the mean value of the longitudinal diameter and transverse diameter is used as the skin test reaction diameter of the injection sample at this point....
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com