Antituberculosis vaccine as well as preparation method and application thereof
A tuberculosis and vaccine technology, applied in the field of tuberculosis vaccine, can solve the problems of being unsuitable for immunocompromised patients and ineffective in adults, and achieve the effects of effective and durable immunity, fast onset of action, and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention provides a kind of preparation method of anti-tuberculosis vaccine, comprises the following steps:
[0042] a. Obtain mycobacterial bacterial strains, inoculate and culture them to the logarithmic growth phase, add resuspension medium, homogenize, sieve, and obtain single-celled mycobacterial cells;
[0043] b. The anti-tuberculosis vaccine is obtained by irradiating mycobacterium single-cell thalline with low dose of radiation, intermittently, uniformly and circularly.
[0044] Wherein, in the preparation method of the above-mentioned anti-tuberculosis vaccine, the resuspension medium described in step a is phosphate buffered saline (PBST) with Tween added. Further, the resuspension medium is PBS containing 0.05%-0.1% Tween 80.
[0045] Wherein, in the preparation method of the above-mentioned anti-tuberculosis vaccine, the concentration of mycobacterium single-cell thalline described in step a is 10 6 / ml~10 8 / ml.
[0046] Wherein, in the preparatio...
Embodiment 1
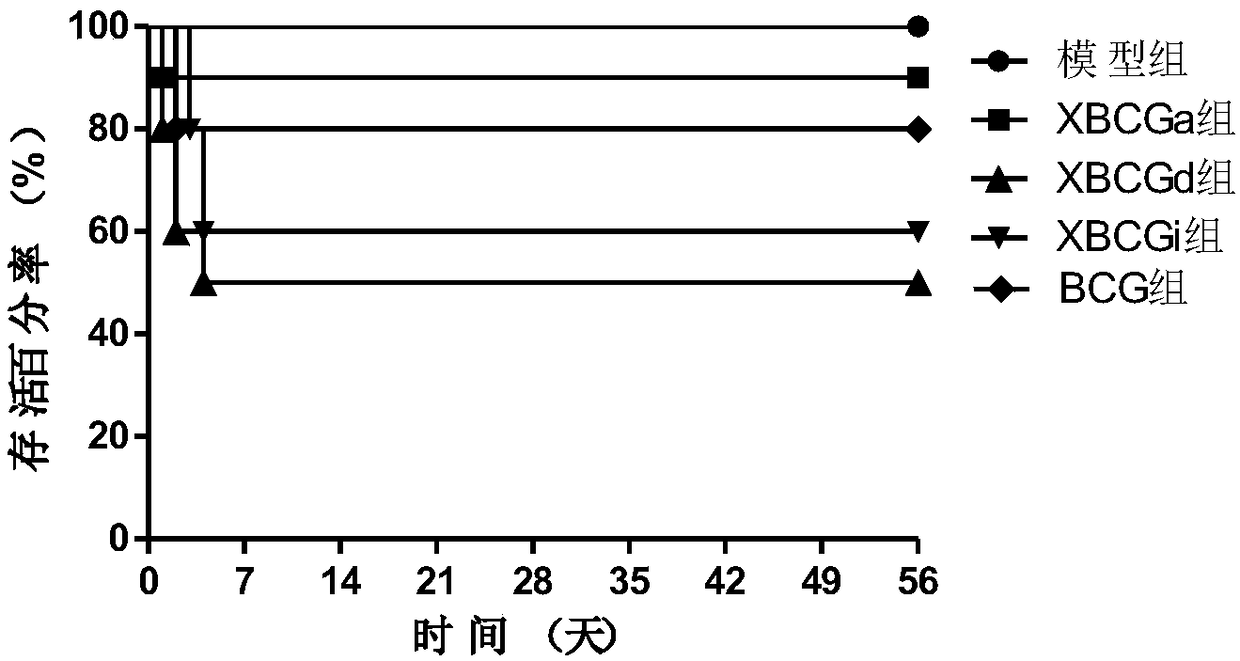
[0073] Example 1 Screening of the irradiation mode of anti-tuberculosis vaccine
[0074] C57BL / 6 mice were selected and divided into model group (PBS), XBCGa group (uniform irradiation; the dose rate was constant at 19Gy / min, 20min each time, 9 times in total, and the total amount of irradiation was 3420Gy), XBCGd group (decreasing radiation irradiation; the dose rate decreased from 35Gy / min to 19Gy / min and 12Gy / min in turn, each dose rate was irradiated 3 times, 20min each time, a total of 9 times, the total amount of irradiation was 3960Gy), XBCGi group (increased irradiation ; The dose rate increased from 12Gy / min to 19Gy / min and 35Gy / min, each dose rate was irradiated 3 times, 20min each time, a total of 9 times, the total amount of irradiation was 3960Gy), BCG group (BCG, Commercially available), 10 rats in each group, subcutaneously immunized once, injection volume 0.1mL (about 10 6 CFU / mL). After 4 weeks of immunization, the virus was challenged intravenously with fre...
Embodiment 2
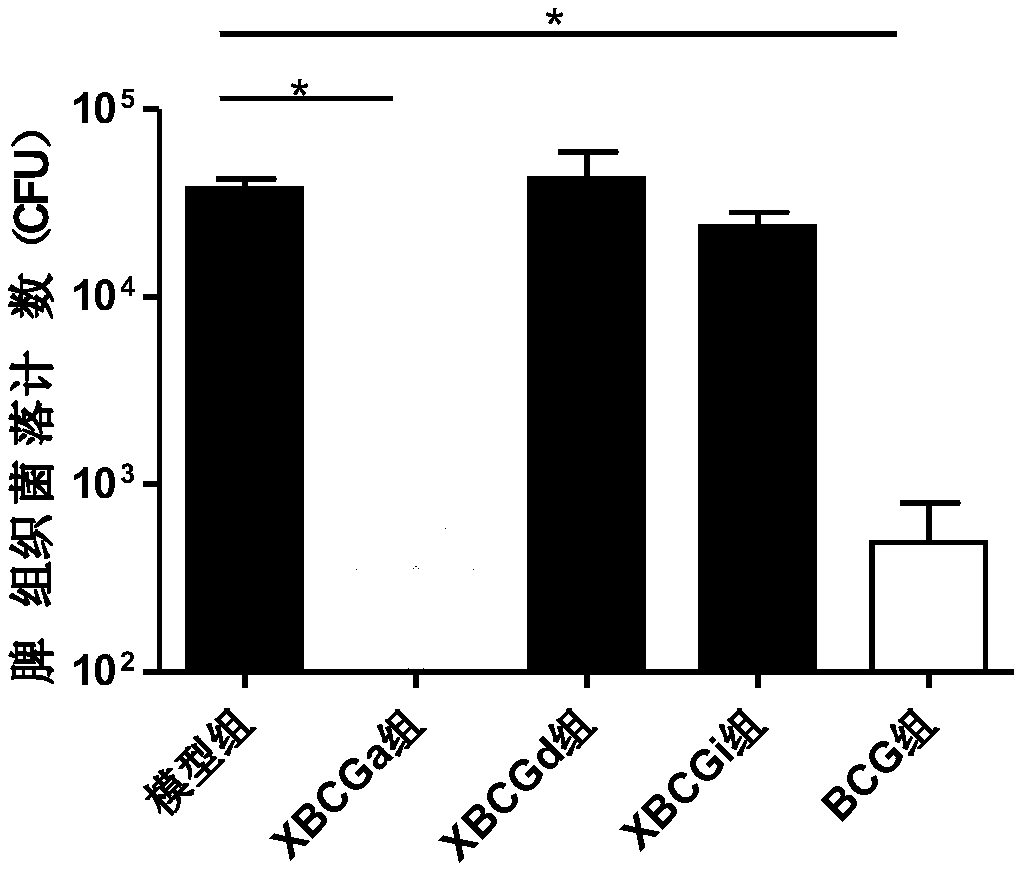
[0078] Example 2 Observation of Immune Protection by Irradiation of Anti-TB Vaccine of the Present Invention
[0079] C57BL / 6 mice were selected and divided into model group (PBS), XBCGa group (uniform irradiation), XBCGd group (decreasing irradiation), XBCGi group (increasing irradiation), BCG group, 10 mice in each group, subcutaneously immunized for 1 times, injection volume 0.1mL about 10 5 CFU. After 4 weeks of immunization, the virus was challenged intravenously with freshly cultured BCG, and the challenge dose was 10 6 CFU, the spleen tissue homogenate was collected and counted with 7H10 at the 4th week after challenge.
[0080] The result is as figure 2 Shown:
[0081] The experiment showed that compared with the model group, the CFU of the XBCGa group and the BCG group decreased significantly at the 4th week, while the XBCGd group and the XBCGi group had no significant change compared with the model group. The results suggested that the anti-tuberculosis vaccine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com