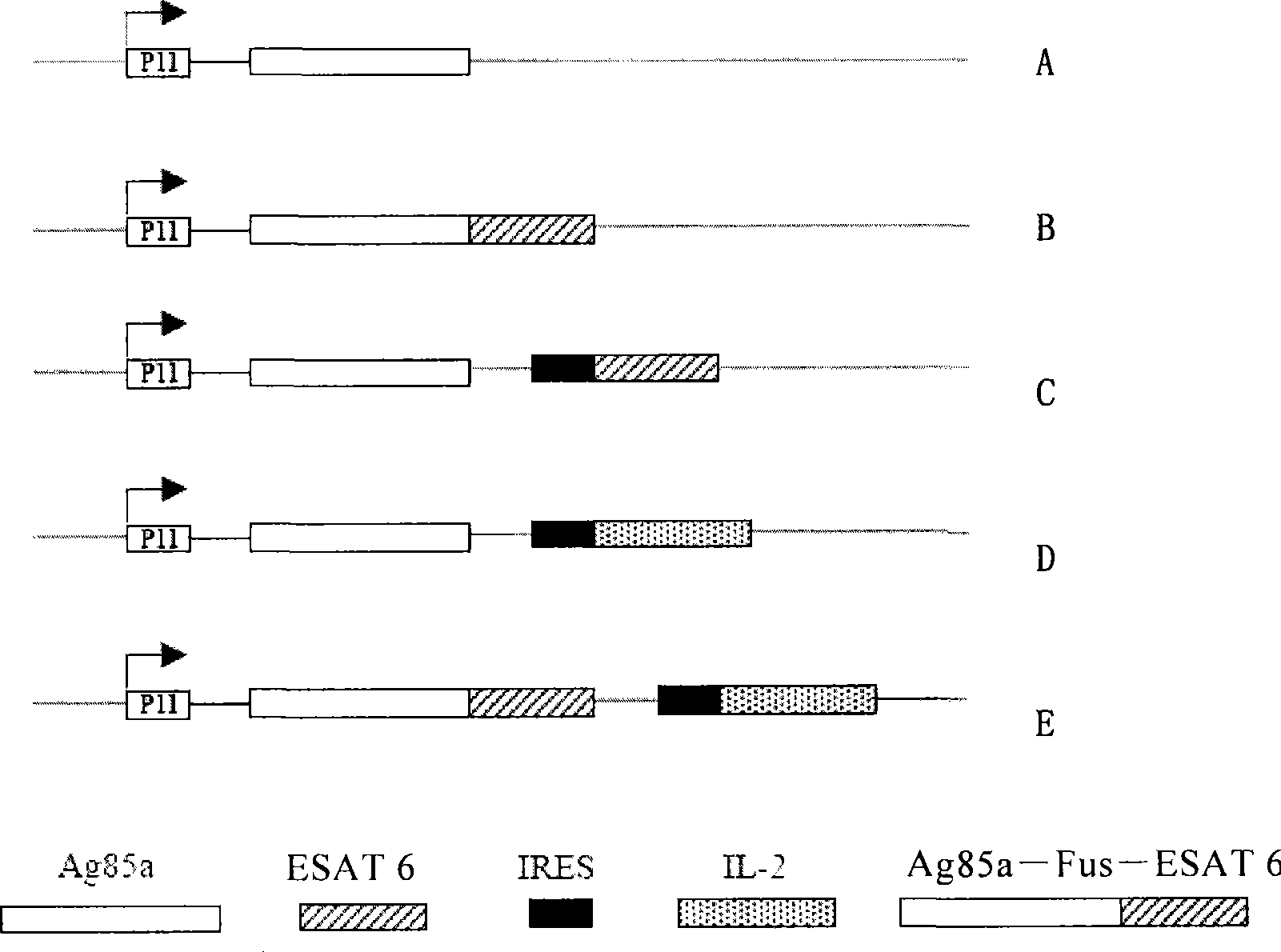
Preparation of novel tuberculosis vaccine and use thereof
A technology of Mycobacterium tuberculosis and virus vectors, applied in the field of bioengineering and immunization, can solve the problems that cannot be applied to immunodeficiency patients, adults have no protective effect, and the immune effect is insufficient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
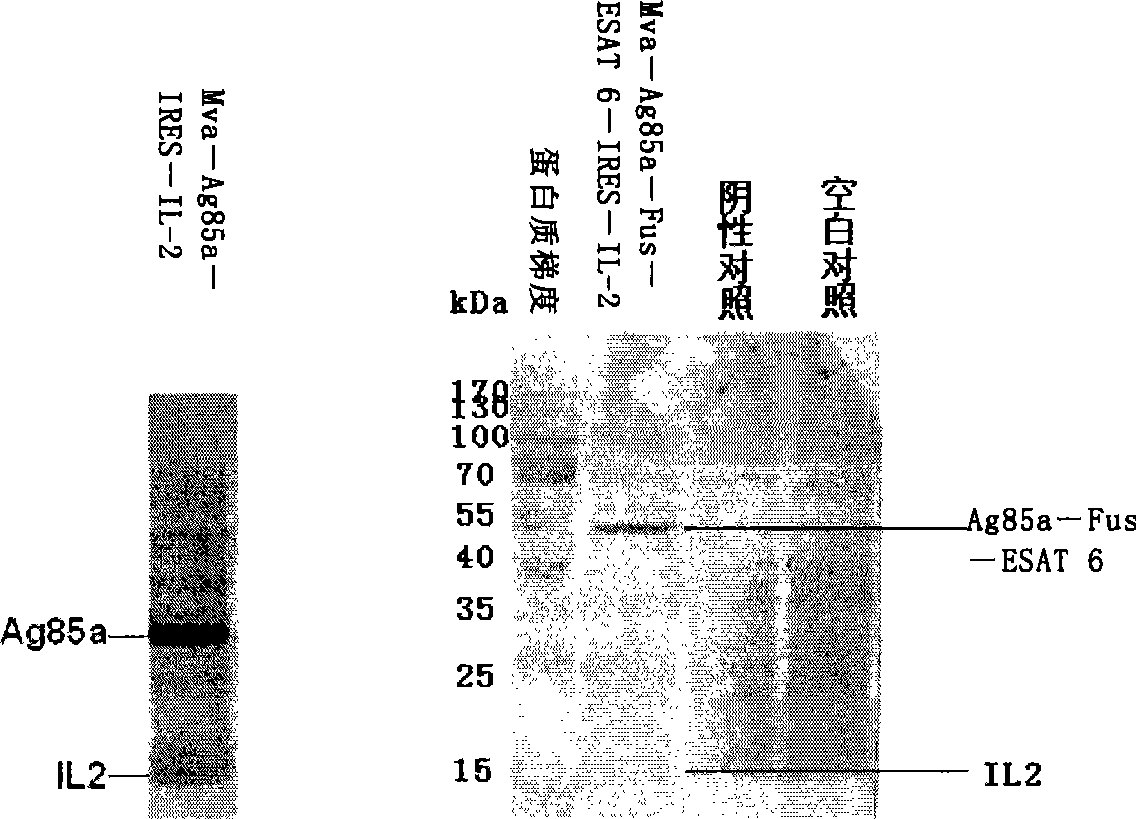
Image
Examples
Embodiment 1
[0110] The construction of embodiment 1.pIL-2 recombinant plasmid
[0111] 1. Obtain and amplify IL-2 (480bp) fragment by PCR
[0112] IL-2 forward primer: gcgcatgcatgtacaggatgcaactcctg (SEQ ID NO: 10)
[0113] IL-2 reverse primer: gcaagcttacttaattatcaagtcagtg (SEQ ID NO: 11)
[0114] Template: human IL-2 DNA fragment or human cDNA prepared by conventional methods.
[0115] PCR reaction system: (μl)
[0116] template forward primer reverse primer 10×Tag buffer 10mM dNTPs 25mM MgCl 2 wxya 2 o Tag enzyme 1 1 1 5 1 3 37 1
[0117] PCR reaction conditions: 94°C for 5 minutes → (94°C for 30 seconds → 56°C for 30 seconds → 72°C for 90 seconds) 30 cycles → 72°C for 10 minutes
[0118] Recovery of PCR products: PCR product rapid purification kit (Broadtech) was used for gel cutting and recovery to obtain 40 μl of IL-2 (480 bp) fragments.
[0119] 2. Insert the target fragment into the expression plasmid to construct the recombinant plasmid...
Embodiment 2
[0133] Construction of embodiment 2.pESAT6 recombinant plasmid
[0134] 1. Obtain and amplify ESAT6 (380bp) fragment by PCR
[0135] ESAT6 forward primer (SEQ ID NO: 12): gcgcatgcatggatgcaatgaagagagggctctgctgtgtgctgctgctgtgtggagcagtcttcgtttcgcccagcacagagcagcagtggaatttc
[0136]ESAT6 reverse primer (SEQ ID NO: 13): gcaagcttcgttgccctatgcgaacatcc
[0137] Template: Mycobacterium tuberculosis H37Rv genome
[0138] PCR reaction system: (μl)
[0139]
template
forward primer
reverse primer Contains MgSO 4 10×
Pfu buffer
10mM dNTPs
wxya 2 o
Pfu enzyme 1 1 1 5 1 40 1
[0140] PCR reaction conditions: 95°C for 5 minutes → (95°C for 30 seconds → 60°C for 30 seconds → 72°C for 60 seconds) 30 cycles → 72°C for 10 minutes
[0141] Recovery of PCR products: PCR product rapid purification kit (Broadtech) was used for gel cutting and recovery to obtain 40 μl of ESAT6 (380 bp) fragments.
[0142] In addition, the 380bp of ESAT...
Embodiment 3
[0159] Example 3. Construction of pIRES-IL-2 recombinant plasmid
[0160] 1. Obtain and amplify the IRES (650bp) fragment by PCR
[0161] IRES forward primer (SEQ ID NO: 14): GCGTCGACCCGAAGTAACTTAGAAGCTG
[0162] IRES reverse primer (SEQ ID NO: 15): GCGCATGCATGTTTGATTGTGTTGAGGG
[0163] Template: Commercially available plasmid containing ribosome entry site IRES
[0164] PCR reaction system: (μl)
[0165]
template
forward primer
reverse primer
Contains MgSO 4 10 x Pfu buffer
liquid
10mM dNTPs
wxya 2 o
Pfu enzyme
1 1 1 5 1 40 1
[0166] PCR reaction conditions;
[0167] 95°C for 5 minutes → (95°C for 30 seconds → 65°C for 30 seconds → 72°C for 1.5 minutes) 30 cycles → 72°C for 10 minutes
[0168] Recovery of PCR products: PCR product rapid purification kit (Broadtech) was used for gel cutting and recovery to obtain 50 μl of IRES (650 bp) fragments.
[0169] 2. Insert the target fragment into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com