TB diagnostic based on antigens from M. tuberculosis
a technology of tuberculosis and diagnostics, applied in the field of new antigens, can solve the problems of inability to demonstrate significant protection in clinical trials in developing countries, inability to approve bcg, and inability to demonstrate a specific long-term protective immune response with the potency of bcg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0173] Identification of Single Culture Filtrate Antigens Involved in Protective Immunity
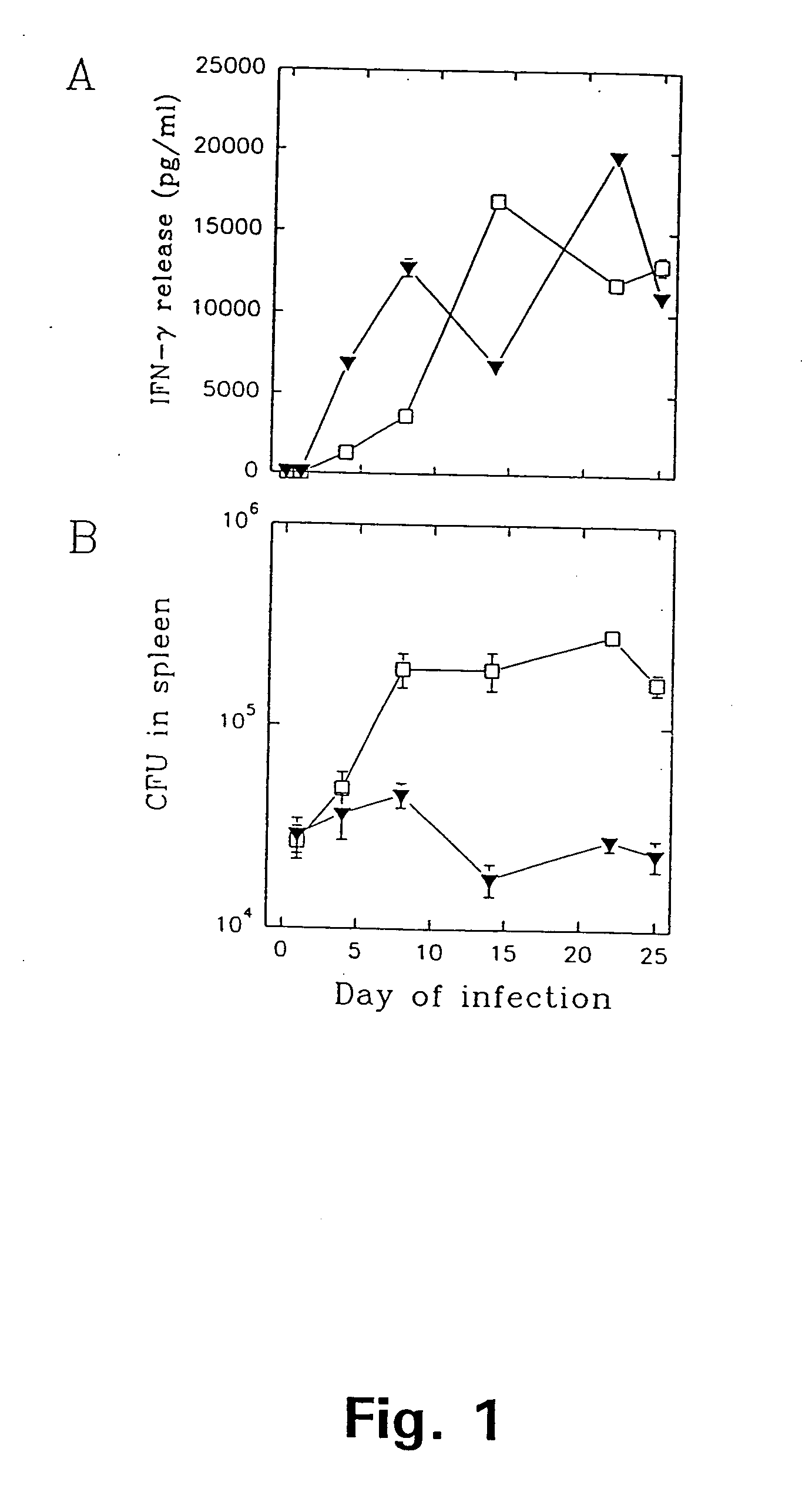
[0174] A group of efficiently protected mice was generated by infecting 8-12 weeks old female C57Bl / 6j mice with 5.times.10.sup.4 M. tuberculosis i.v. After 30 days of infection the mice were subjected to 60 days of antibiotic treatment with isoniazid and were then left for 200-240 days to ensure the establishment of resting long-term memory immunity. Such memory immune mice are very efficiently protected against a secondary infection (FIG. 1). Long lasting immunity in this model is mediated by a population of highly reactive CD4 cells recruited to the site of infection and triggered to produce large amounts of IFN-.gamma. in response to ST-CF (FIG. 1) (Andersen et al. 1995).
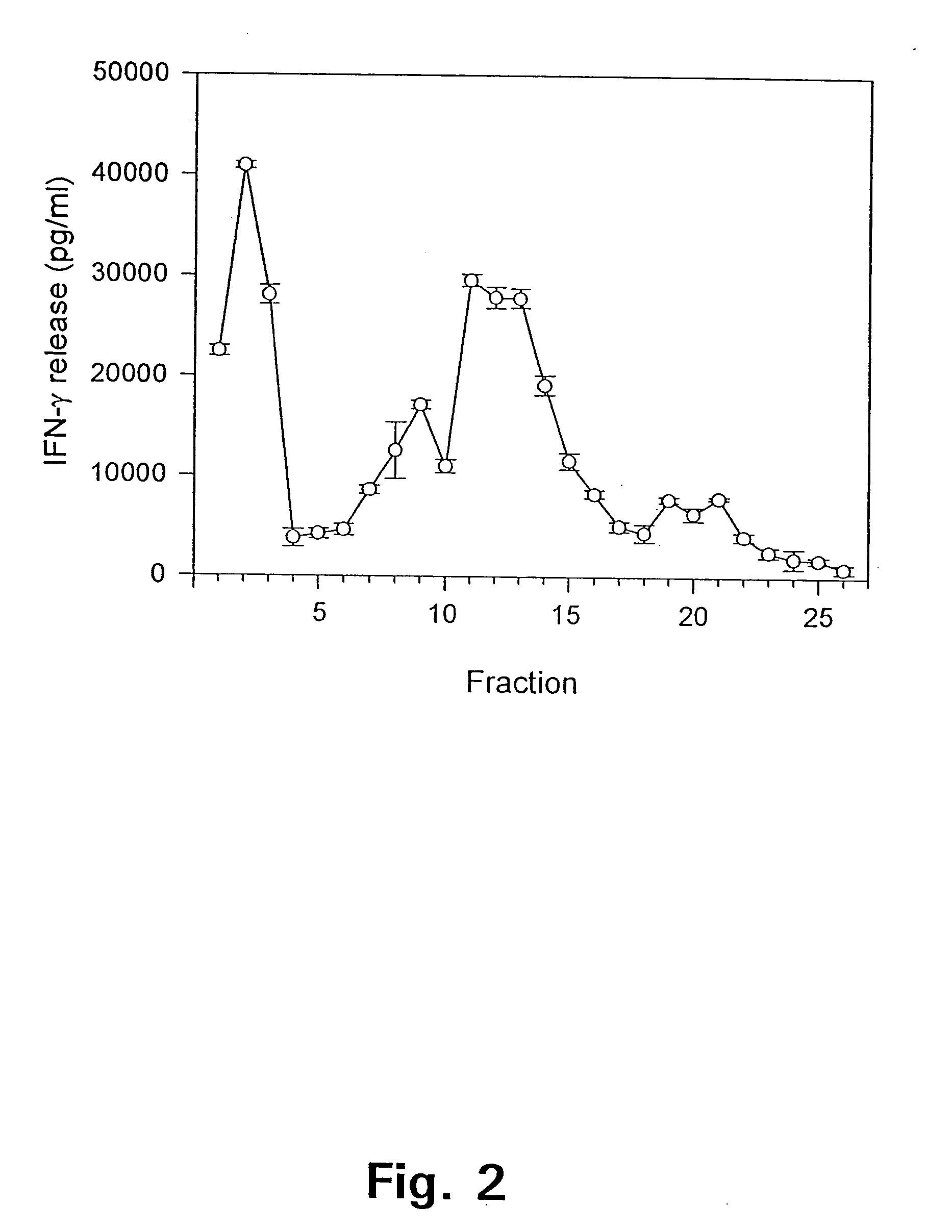
[0175] We have used this model to identify single antigens recognized by protective T cells. Memory immune mice were reinfected with 1.times.10.sup.6 M. tuberculosis i.v. and splenic lymphocytes were harvested at day 4-6 of ...
example 2
[0176] Cloning of Genes Expressing Low Mass Culture Filtrate Antigens
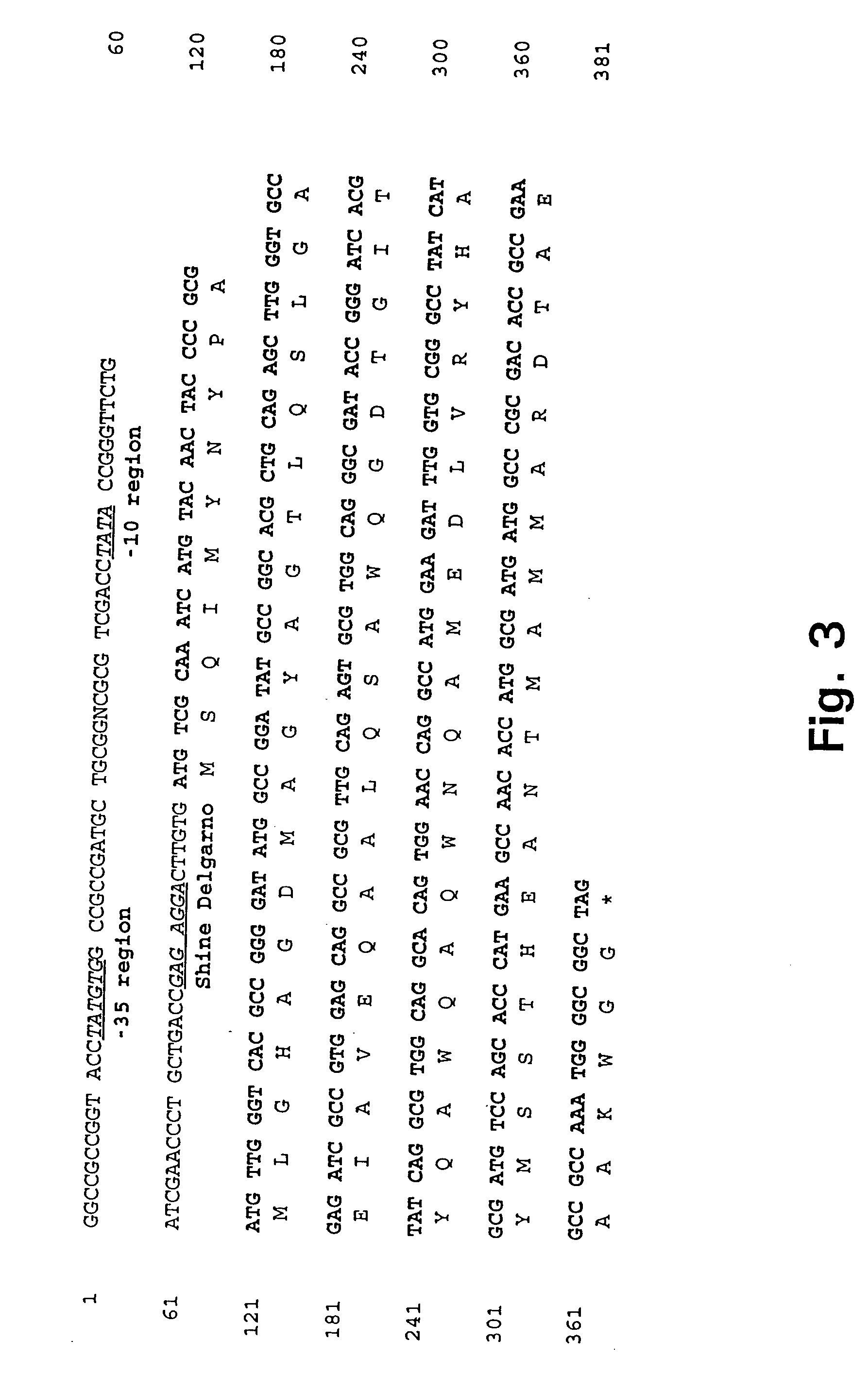
[0177] In example 1 it was demonstrated that antigens in the low molecular mass fraction are recognized strongly by cells isolated from memory immune mice. Monoclonal antibodies (mAbs) to these antigens were therefore generated by immunizing with the low mass fraction in RIBI adjuvant (first and second immunization) followed by two injections with the fractions in aluminium hydroxide. Fusion and cloning of the reactive cell lines were done according to standard procedures (Kohler and Milstein 1975). The procedure resulted in the provision of two mAbs: ST-3 directed to a 9 kDa culture filtrate antigen (CFP9) and PV-2 directed to a 7 kDa antigen (CFP7), when the molecular weight is estimated from migration of the antigens in an SDS-PAGE.
[0178] In order to identify the antigens binding to the Mab's, the following experiments were carried out:
[0179] The recombinant .lambda.gt11 M. tuberculosis DNA library constructed b...
example 2a
[0214] Identification of Antigens which are not Expressed in BCG Strains.
[0215] In an effort to control the treat of TB, attenuated bacillus Calmette-Gurin (BCG) has been used as a live attenuated vaccine. BCG is an attenuated derivative of a virulent Mycobacterium bovis. The original BCG from the Pasteur Institute in Paris, France was developed from 1908 to 1921 by 231 passages in liquid culture and has never been shown to revert to virulence in animals, indicating that the attenuating mutation(s) in BCG are stable deletions and / or multiple mutations which do not readily revert. While physiological differences between BCG and M. tuberculosis and M. bovis has been noted, the attenuating mutations which arose during serial passage of the original BCG strain has been unknown until recently. The first mutations described are the loss of the gene encoding MPB64 in some BCG strains (Li et al., 1993, Oettinger and Andersen, 1994) and the gene encoding ESAT-6 in all BCG strain tested (Harb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com