High throughput method for screening for homologous t cells and epitope reactivity in primary human cells
A cell and antigen technology, applied in the direction of cell culture active agents, biochemical equipment and methods, biological agents to remove bad cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
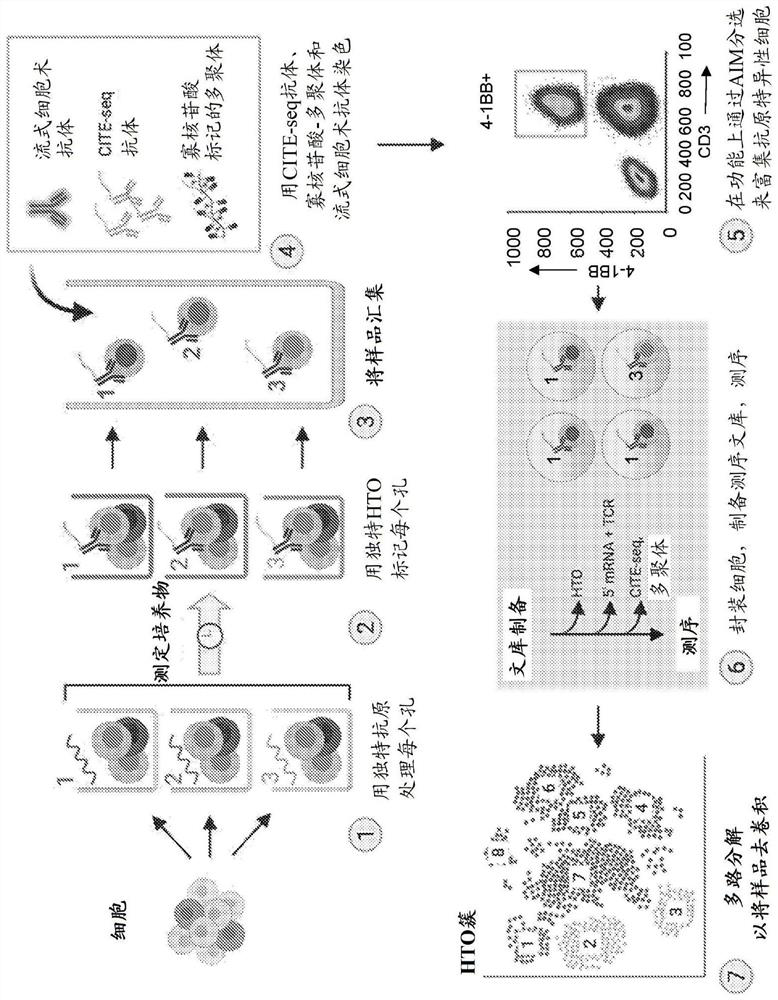
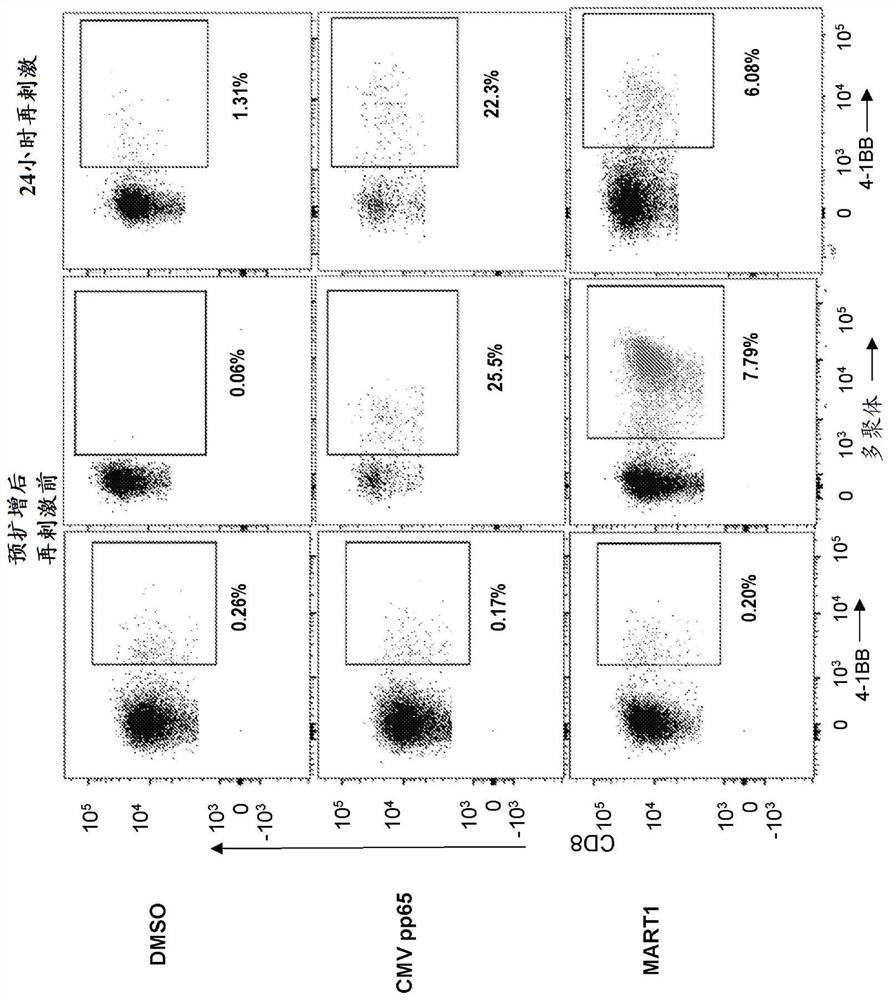
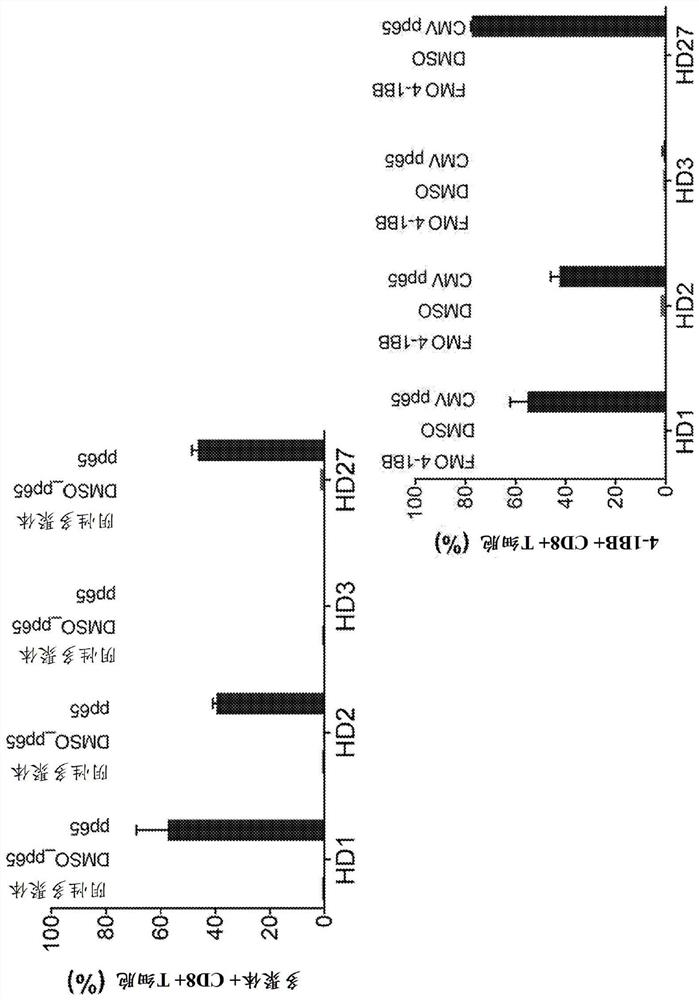
[0156] Embodiment 1. A method for identifying a T cell receptor (TCR) alpha and / or beta chain sequence of a TCR that recognizes an epitope of interest comprising sorting from a pool of T cells with oligonucleotides A population of T cells labeled with a conjugated antibody, wherein the oligonucleotide tag comprises a sequence associated with a unique epitope, antigen or pool of antigens.
Embodiment approach 2
[0157] Embodiment 2. The method of embodiment 1, said method comprising the step of determining the sequence of said oligonucleotide tag after sorting, thereby determining the activation of said T tagged with said oligonucleotide-conjugated antibody Said epitope, antigen or pool of antigens of a cell.
Embodiment approach 3
[0158] Embodiment 3. The method of embodiment 1 or embodiment 2, said method comprising one or more of the following steps prior to said sorting step:
[0159] For example, establishment of multiple cultures from peripheral blood mononuclear cell (PBMC) samples in multi-well culture plates, where each culture contains media and cytokines that support the function and growth of antigen-presenting cells (APCs) and T cells,
[0160] delivering a unique antigen or pool of antigens of interest to each of the multiple cultures, thereby establishing unique cultures, e.g., adding a single antigen (or pool of antigens) of interest to the multiple cultures One, e.g. in one well of said culture plate, wherein each culture (well) contains a unique antigen or pool of antigens,
[0161] adding to the unique culture a unique oligonucleotide tag associated with said unique culture, and
[0162] Optional addition of other surface-staining antibodies and multimers that may also contain oligome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com