Method for enhancing antigen response of dendrite cells by modifying dendrite cells
An antigen and cell technology, applied to cells modified by introducing foreign genetic material, other methods of inserting foreign genetic material, recombinant DNA technology, etc., can solve problems such as lack of antigen effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
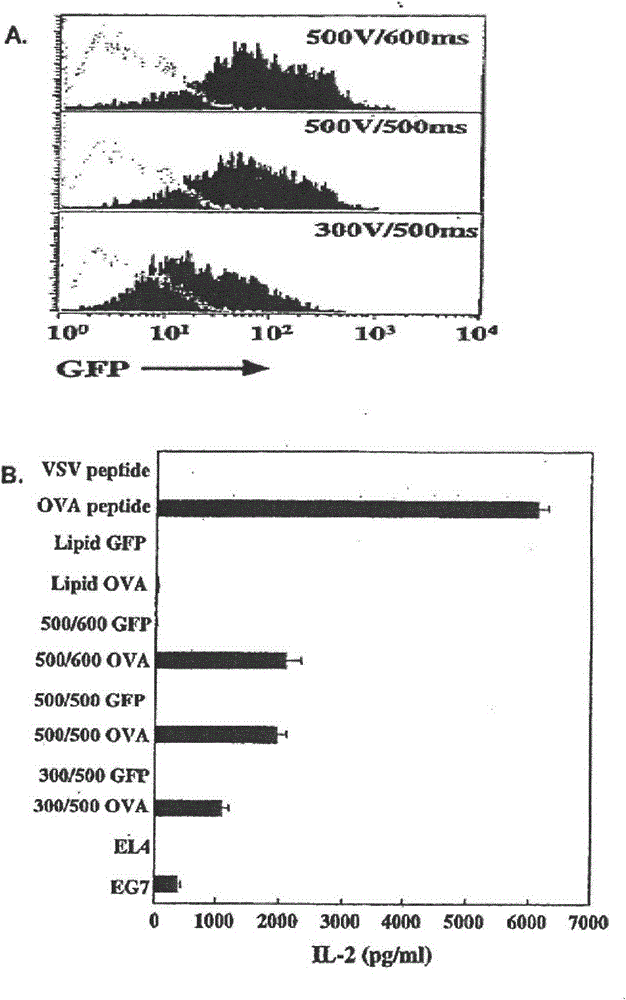
[0031] MHC class I presentation of antigen: GFP mRNA was introduced into DC by electroporation, and the expression of GFP was detected by flow cytometry after 24 hours. Electroporation of mRNA is a method for efficiently expressing and presenting antigens in human DCs. like figure 1 As shown in A, GFP mRNA efficiently transfected bone marrow-derived mouse DCs. Voltage and duration of electroporation affect transfection efficiency. like figure 1 B demonstrates the presentation of major MHC class I-restricted OVA peptides in DCs that have been electroporated with OVA mRNA or GFP mRNA. Presentation assays were performed with RF33.70T-hybridoma cells. Transfection of DCs with OVA was performed in the presence of DMRIE-C lipids, or pulsed with MHC class I-restricted OVA or VSV peptides. like figure 1 B shows the process of electroporation of mRNA encoding chicken OVA cDNA into DCs and presentation of major OVA epitopes to corresponding T-hybridoma cells. The efficiency of OV...
Embodiment 2
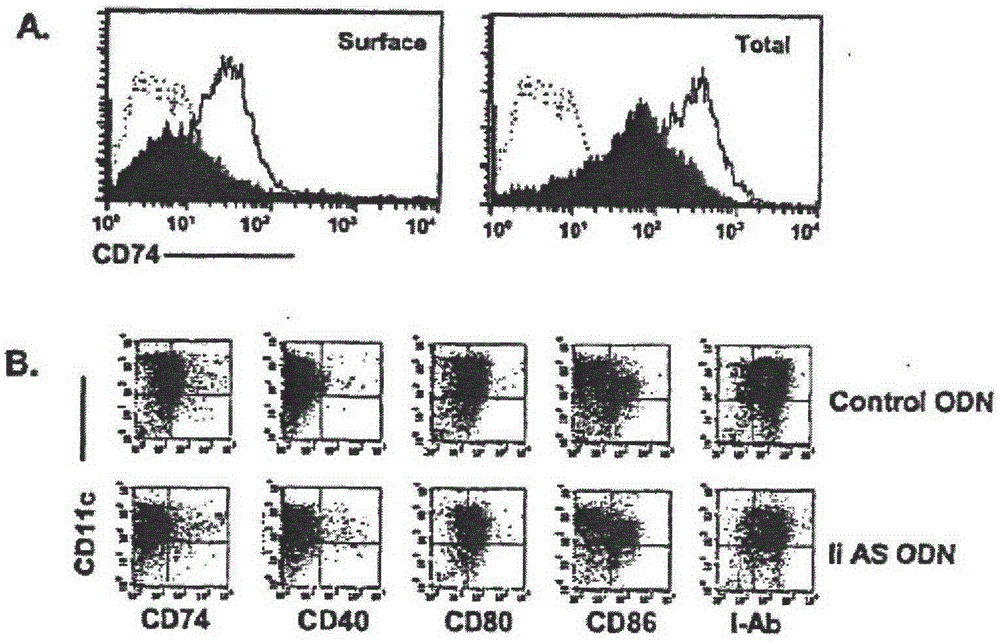
[0033] Treatment of DCs with antisense oligonucleotides to suppress their invariant strand expression: Two phosphorylation-modified antisense oligonucleotides (ODNs) were used to suppress the expression of li in mouse DCs. On day 7, OVA mRNA and 50 nM li AS ODN (AE40) or control ODN (SE40) were introduced into DCs by electroporation. After 48 hours, the cells were stained with FITC-labeled anti-mouse CD74(li) antibody to detect the expression of li on the cell surface. The result is as figure 2 As shown in A. Black histograms show the results of li AS ODN treatment, and blank histograms represent the results of control ODN treatment. Dashed histograms represent DCs stained with FITC-labeled antibodies of the same type. Cells were permeabilized prior to staining with antibodies to detect total li expression. figure 2 A shows that the expression of li(CD74) on the surface of DCs was significantly reduced after co-incubation with antisense oligonucleotides, but not in the c...
Embodiment 3
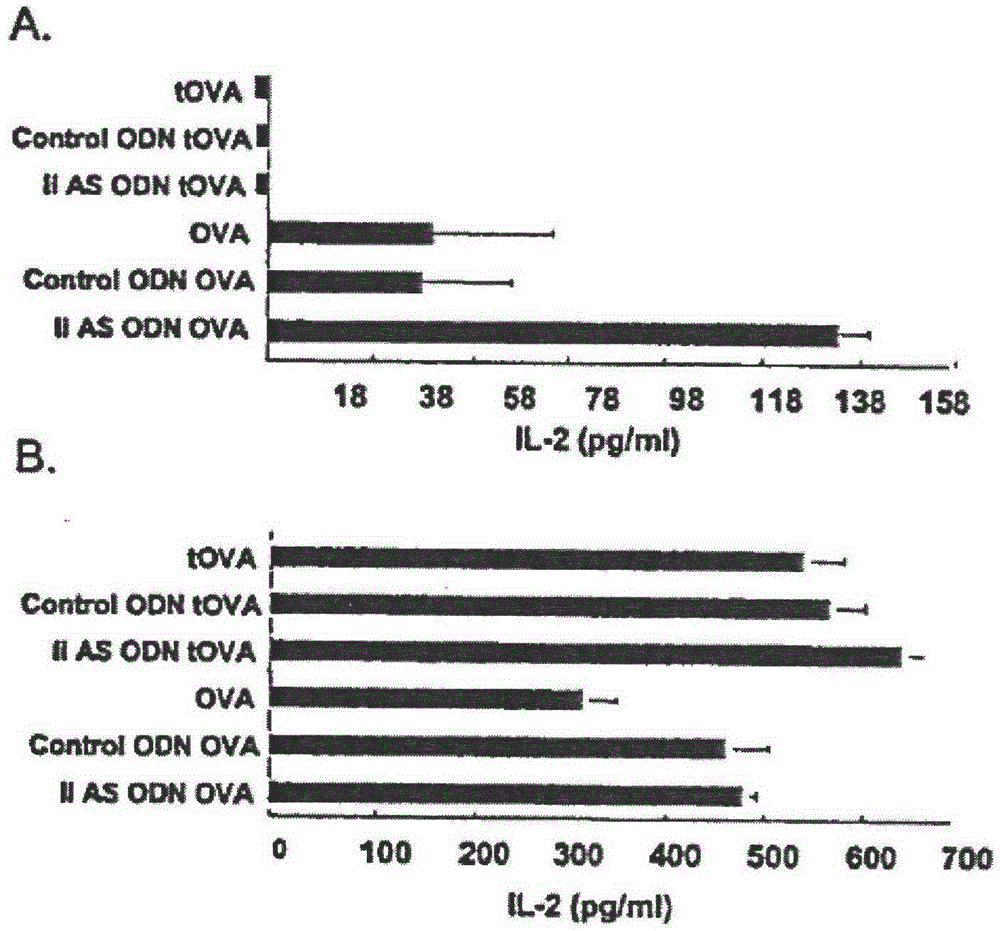
[0035] Presentation of MHC class I and class II OVA epitopes: Transfection of DCs with OVA mRNA inhibited li synthesis, thereby enhancing MHC class II presentation of OVA. The DCs used were transfected with OVA mRNA or truncated OVA mRNA (tOVA) in which the first 40 nucleic acids were cleaved. DCs transfected with OVA mRNA were also incubated with AE40 or SE40. The processing and presentation of class I and class II epitopes by OVA mRNA-transfected DCs was determined by the T hybridoma cells specific for the respective epitopes used. C57BL / 6(H-2B) mice present an H-2Kb class I-restricted epitope, as well as an I-Ab-restricted class II epitope. like image 3 As shown in A, the expression of class II antigen epitopes was not detected in the tOVA group. In contrast, for native and secreted OVA, class II epitopes are processed for presentation to class II-restricted T-hybridoma cells. Co-incubation of li antisense primers with OVA mRNA-transfected DCs enhanced the expression o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com