Preparation method and application of antigen reactive T cell based on RNA vaccine
A reactive and cell-based technology, applied in the direction of genetically modified cells, blood/immune system cells, biochemical equipment and methods, etc., can solve the problems of long polypeptide vaccine preparation cycle, low T cell expansion efficiency, low degree of cell activation, etc. problems, to achieve the effect of short preparation cycle, high preparation success rate and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] A method for preparing antigen-reactive T cells based on RNA vaccine, comprising the following steps:
[0044] Construct the RNA vaccine in vitro expression vector, and obtain the RNA vaccine after plasmid linearization and in vitro transcription; wherein, the construction of the RNA vaccine includes direct synthesis and construction of the expression vector for in vitro transcription. This application uses the construction of the expression vector for in vitro transcription, which has a short production cycle. Low cost, large-scale production and other advantages; mammalian expression vector can be pcDNA TM 6. pcDNA TM 5. pCEP4, pcDNA3.1, etc., this application is preferably pcDNA3.1, which has the advantages of being applicable to high-level and constitutive expression in various mammalian cell lines.
[0045] T cells and RNA vaccine-modified DC cells were obtained based on the collected PBMCs; among them, the common methods for RNA vaccines to enter DC cells include...
Embodiment 1
[0048] Construct RNA vaccine in vitro expression vector, and obtain RNA vaccine after plasmid linearization and in vitro transcription:
[0049] (1) At room temperature, take a 200ul PCR tube, add 15ul of double-distilled water, 2ul of 10X buffer, 1ug of plasmid (2ul), 1ul of XhoI enzyme in turn, shake and mix, and incubate at 37°C for 5min. Plasmid linearization;
[0050] (2) The linearized plasmid was purified and recovered using a PCR purification kit (qiagen, 28104);
[0051] (3) At room temperature, add the linearized pcDNA3.1+ plasmid, T7 transcription premix, T7 RNA polymerase, add double distilled water to the system of 20ul, 37°C, warm bath for 1h, and perform in vitro transcription of the plasmid ;
[0052] (4) The in vitro transcribed RNA vaccine was purified and recovered using an RNA purification kit (qiagen, 74134).
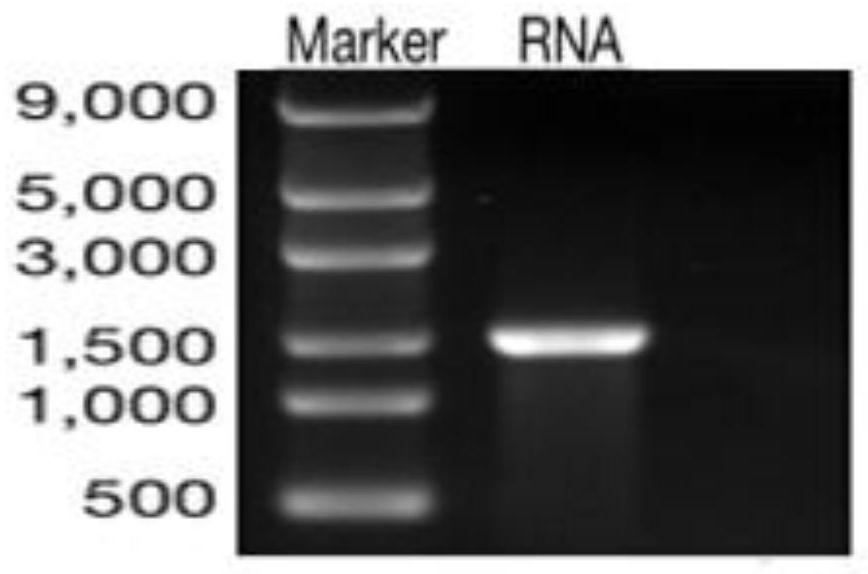
[0053] The obtained RNA vaccine was tested for integrity.
[0054] The present invention has been tested for integrity: as figure 1 The frag...
Embodiment 2
[0056] Obtain T cells and RNA vaccine-modified DC cells based on harvested PBMC:
[0057] (1) On day 0, 80-100 ml of peripheral blood mononuclear cells were collected by an apheresis machine. PBMCs were separated by Ficoll-Histopaque, adhered to the wall for 2 hours, and non-adherent cells (T cells) were collected and frozen for future use. Adherent cells (DC cells) are added to the DC culture medium for the preparation of RNA vaccines;
[0058] (2) On the second day, absorb the DC culture supernatant, centrifuge for 5 minutes, remove half the volume of medium, add a corresponding volume of fresh medium and supplement cytokines to maintain a certain solubility of cytokines (rhGM-CSF and IL-4) ;
[0059] (3) On day 4, DC cells were induced to mature. Add TNF-α, IL-1b, IL-6 to induce DC cell maturation overnight;
[0060] (4) On the 5th day, mature DC cells were collected, and RNA vaccine (DC cells modified by RNA vaccine) was electroporated.
[0061] Take 10ml of whole blo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com