Use of the cd2 signaling domain in second-generation chimeric antigen receptors
A chimeric antigen receptor, signal transduction technology, applied in the field of ICOS, lymphocyte function-related resistance, can solve problems such as quantitative and qualitative differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
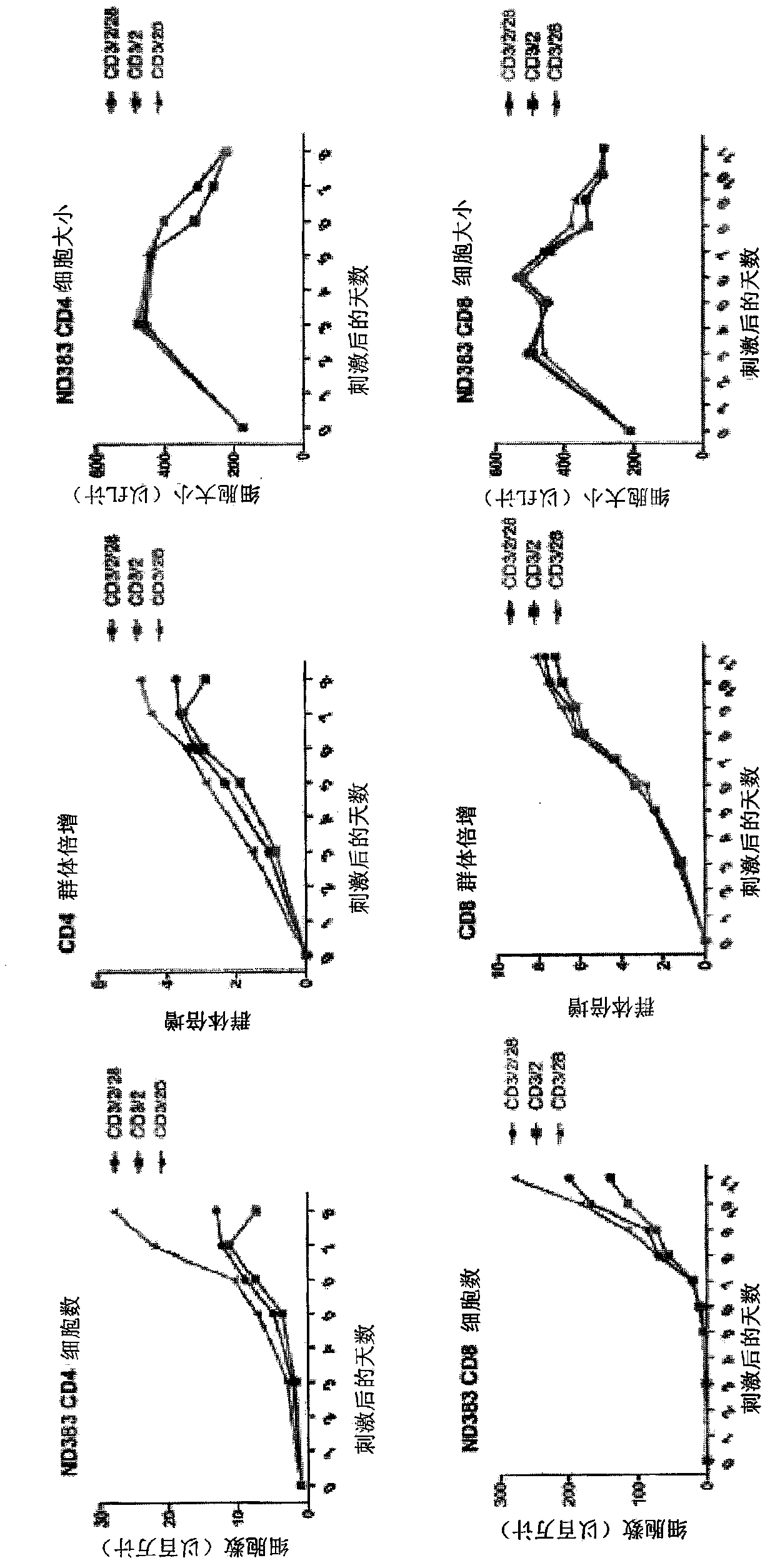
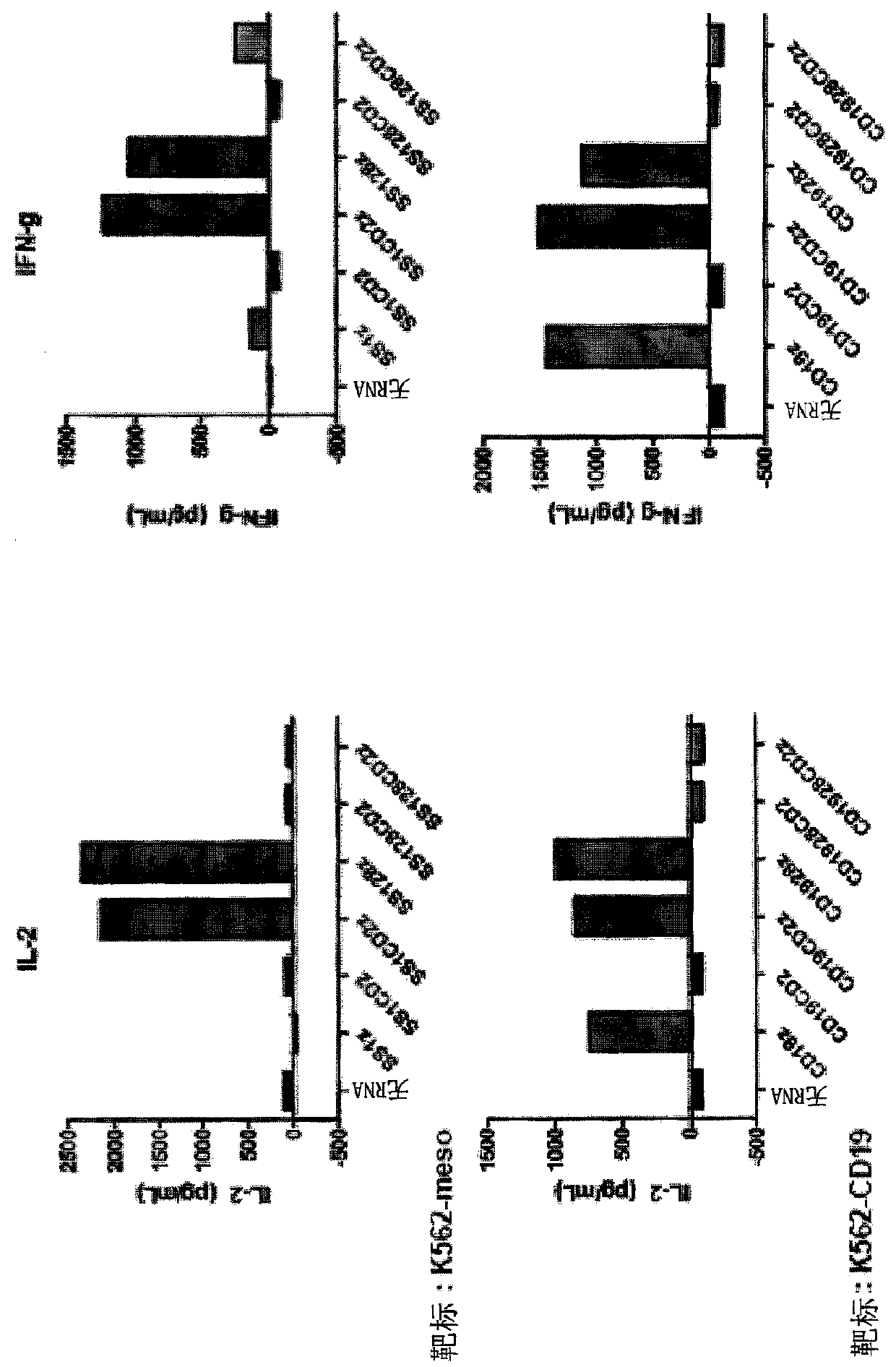
[0255] Example 1: Use of the CD2 Signaling Domain in Second Generation Chimeric Antigen Receptors
[0256] Incorporation of co-stimulatory domains into chimeric antigen receptors increases cytokine production and target cell killing. CD2 is a co-stimulatory molecule that can affect calcium-mediated signaling. Here it was examined whether inclusion of the CD2 intracellular domain in the CAR affects calcium influx / signaling and cytokine production.
[0257] The materials and methods used in these experiments are now described.
[0258] CD2-containing CAR
[0259] Cloning of the CD2 co-stimulatory domain into various CD19 or mesothelin-specific CARs. E.g, figure 1 The nucleotide sequence of the anti-CD19-CD2z CAR is shown (SEQ ID NO: 1).
[0260] T cells
[0261] Blood samples were obtained from the Human Immunology Core at the University of Pennsylvania. Using RosetteSep kit (stem cell technology), peripheral blood CD4 + and CD8 + T cell negative isolation. Cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com