BCMA-based (B cell maturation antigen-based) chimeric antigen receptor and preparation method and application thereof
A chimeric antigen receptor and antibody technology, which is applied in the fields of biochemical equipment and methods, antibody medical components, chemical instruments and methods, etc., can solve the problem that the killing effect of tumor cells is not long-lasting, the killing power cannot be exerted, and the tumor cells cannot be fully exerted. Killing effect and other issues, to achieve the effect of good tumor targeting, strong killing ability, and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
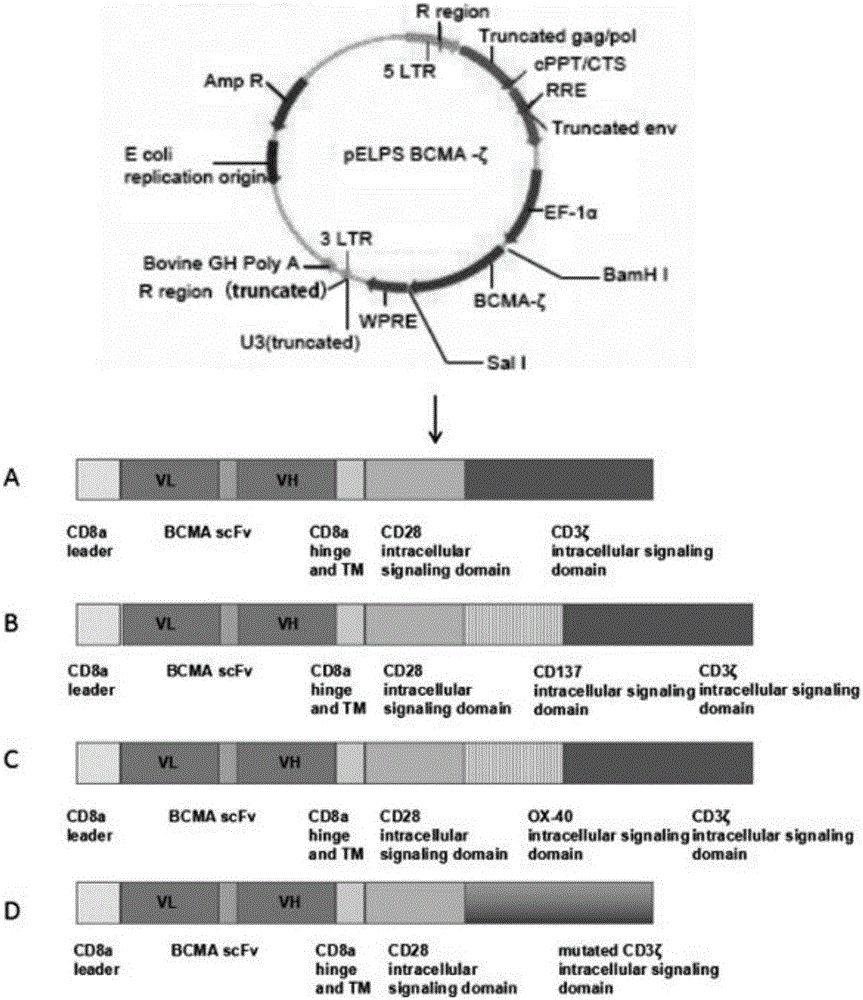
[0051] Specifically, in an embodiment of the present invention, the method for preparing the DNA sequence of the BCMA-based chimeric antigen receptor described in the present invention includes the following steps: extracting mRNA in PBMC, and obtaining cDNA through Vazyme reverse transcription kit; and Using the obtained cDNA as a template, primers were designed, and the guide sequence encoding CD8a (BamHI restriction site was introduced upstream), the hinge region and transmembrane region of CD8a (NheI restriction site was introduced upstream), and the intracellular signal of CD137 were respectively obtained by PCR. domain, the OX40 intracellular signaling domain, the DNA fragment of the CD3ζ intracellular signaling domain (the SalI restriction site is introduced downstream), the intracellular domain of CD3ζmut, and these fragments are combined by overlappingPCR to obtain CD8-CD28- CD3ζ, CD8-CD28-CD137-CD3, CD8-CD28-OX40-CD3ζ, the restriction site NheI / SalI was introduced int...
Embodiment 1
[0070] Example 1 Construction of recombinant vector pELPS-BCMAscFv-CD8-CD28-CD3ζ
[0071] Based on the vector pELPS-19-BB-ζ derived from the patent US_2015_0093822_A1, the restriction endonuclease BamHI / SalI was used for double digestion, and the promega gel recovery kit was used to recover large fragments. The specific method was carried out according to the instructions.
[0072] The Fab sequence of the BCMA antibody was derived from PDB: 4ZFO, using pUC-4ZFO-Fab-H and pUC-4ZFO-Fab-L (sequence synthesized by Genscript) as templates, designed primers, and obtained VH and VL by PCR. A linker was introduced to obtain BCMAscFv by overlappingPCR. The downstream of the BCMAscFv sequence was introduced with restriction site NheI.
[0073] The mRNA in PBMC was extracted, cDNA was obtained by Vazyme reverse transcription kit, and primers were designed using the obtained cDNA as a template, and the CD8a guide sequence (introduced upstream BamHI restriction site), CD8a transmembrane r...
Embodiment 2
[0077] Example 2 Construction of recombinant vector pELPS-BCMAscFv-CD8-CD28-CD137-CD3ζ
[0078] Based on the vector pELPS-19-BB-ζ derived from the patent US_2015_0093822_A1, the restriction endonuclease BamHI / SalI was used for double digestion, and the promega gel recovery kit was used to recover large fragments. The specific method was carried out according to the instructions.
[0079] The Fab sequence of the BCMA antibody was derived from PDB: 4ZFO, using pUC-4ZFO-Fab-H and pUC-4ZFO-Fab-L (sequence synthesized by Genscript) as templates, designed primers, and obtained VH and VL by PCR. A linker was introduced to obtain BCMAscFv by overlappingPCR. The downstream of the BCMAscFv sequence was introduced with restriction site NheI.
[0080] The mRNA in PBMC was extracted, cDNA was obtained by Vazyme reverse transcription kit, and primers were designed using the obtained cDNA as a template, and the CD8a guide sequence (introduced upstream BamHI restriction site), CD8a transmemb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com