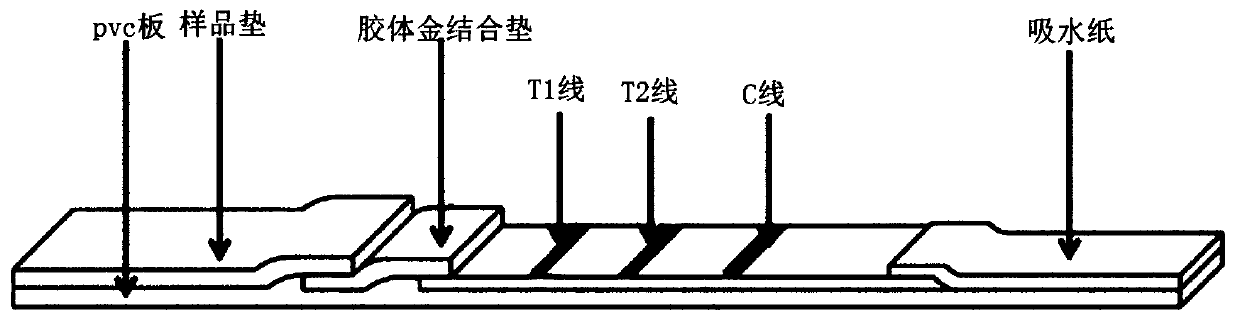
Binding protein combination for detecting plasmodium falciparum HRP2 and plasmodium vivax LDH and preparation method and application thereof
A Plasmodium falciparum and binding protein technology, applied in the field of phage display, can solve the problems of high detection cost, impossibility of popularization and application, and expensive raw materials, and achieve the effect of low production cost, high clinical application value, and low requirements for storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Phage display library panning process for protein reagents targeting P. falciparum L-lactate dehydrogenase and histidine-rich protein 2:
[0042] (1) First use NHS-EZ coupling reagent (purchased from Pierce) to label the target protein with biotin. The effect of biotin labeling was confirmed by detecting the presence of biotin by ELISA method. The biotin-labeled protein was determined by adsorbing the target protein on a 96-well plate (purchased from Nunc Company), and then using streptavidin-coupled horseradish peroxidase (HRP) for biotin detection. The panning process of the phage display library is as follows: Take a 5 μL aliquot of the phage display library containing complexity 10 12 Phage plasmids were mixed with 95 μL of phosphate buffer containing 0.1% Tween 20, and then pre-panned 3 times on a high-binding streptavidin-coated plate (purchased from Pierce Company). 1 hour. Add 100 μL of 100 nM biotinylated protein to the panning wells coated with streptavidin...
Embodiment 2
[0046] The presence of phage of the target protein reagent is detected by ELISA method, and the verification result is further confirmed by sequencing. The process is as follows:
[0047] The phages were recovered from the wells containing the target protein, and the quality control well was used to judge the amplification in the target well. The quality control well indicated that the reagents that bind the target protein were amplified efficiently. To test proteins purchased from display libraries, bound target phage were detected by ELISA. Single ER2738 colonies were purchased from infected cells from the last round of panning and cultured in 96-deep well plates in 100 μL of 2TY containing 100 μg / mL carbenicillin at 37 °C (900 rpm). The cells were picked out after 6 hours of culture in medium. Add a 25 μL aliquot of the culture solution to 200 μL 2TY medium containing carbenicillin, and incubate at 37° C. (900 rpm) for 1 hour. Then add helper phage (10 μL of 1011 / mL), and...
Embodiment 3
[0049] Introduce the specific DNA sequence for expressing the target protein into the target plasmid and transfer it into Escherichia coli for amplification and culture. After extraction and purification, the protein expression is tested by ELISA method. The steps are as follows:
[0050] All specific reagents were then introduced into protein expression vectors for cloning, and the products were determined as capture reagents in ELISA. The DNA coding sequence (SEQ ID NO: 1-4) of the binding protein that reacts with the target protein is amplified by PCR, and the product is treated with Nhel and Pstl restriction endonucleases, and the digested fragment is introduced into the structure containing the expression binding protein and pET11a plasmid with restriction sites. The clones were picked and cultured in 5 ml of LB medium at 37°C and 225 rpm overnight, and the plasmid DNA was extracted with a minipreps (from Qiagen) plasmid extraction kit, followed by sequencing to further c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com