Preparation method and application of collagen scaffold composite bone marrow-derived mesenchymal stem cells (BMSCs)
A technology of bone marrow mesenchymal and collagen scaffolds, applied in the field of obstetrics and gynecology, can solve the problems of inability to promote the growth of scar intima, affect the curative effect, and difficult to locate, so as to increase the thickness of intima and local blood vessel density, and promote scarred uterus Intimal repair, good biocompatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
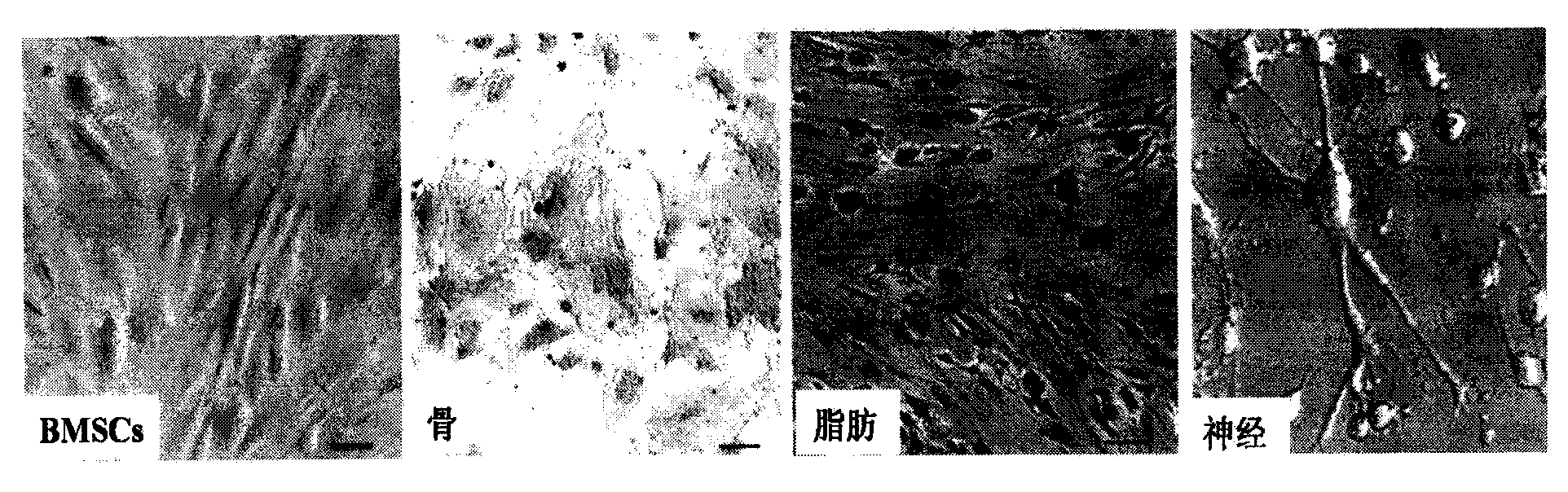
[0036] Composite culture of BMSCs and collagen scaffolds
[0037] 1. Equipment, materials and reagents used
[0038] Ultra clean bench, CO 2 Incubator, inverted microscope, centrifuge, scanning electron microscope, critical point dryer, tissue embedding freezer, paraffin slicer, automatic tissue staining machine, cell culture bottle, 6-well plate, centrifuge tube, L-DMEM culture medium, Fetal bovine serum, penicillin / streptomycin, trypsin, formaldehyde, glutaraldehyde, xylene, concentration gradient ethanol, hematoxylin, eosin, etc.
[0039] 2. BMSCs maintain cell viability
[0040] (1) When cultured until 90% of the adherent cells are confluent, subculture is carried out;
[0041] (2) Remove the old culture medium, add PBS to wash away the residual culture medium, and remove the PBS;
[0042] (3) Add 1ml of trypsin and shake the flask gently;
[0043] (4) Observing under an inverted microscope, it was found that the cytoplasm retracted and the intercellular space increas...
Embodiment 2
[0062] Collagen Scaffold Composite BMSCs Used in the Repair Treatment of Severe Uterine Injury Animal Model
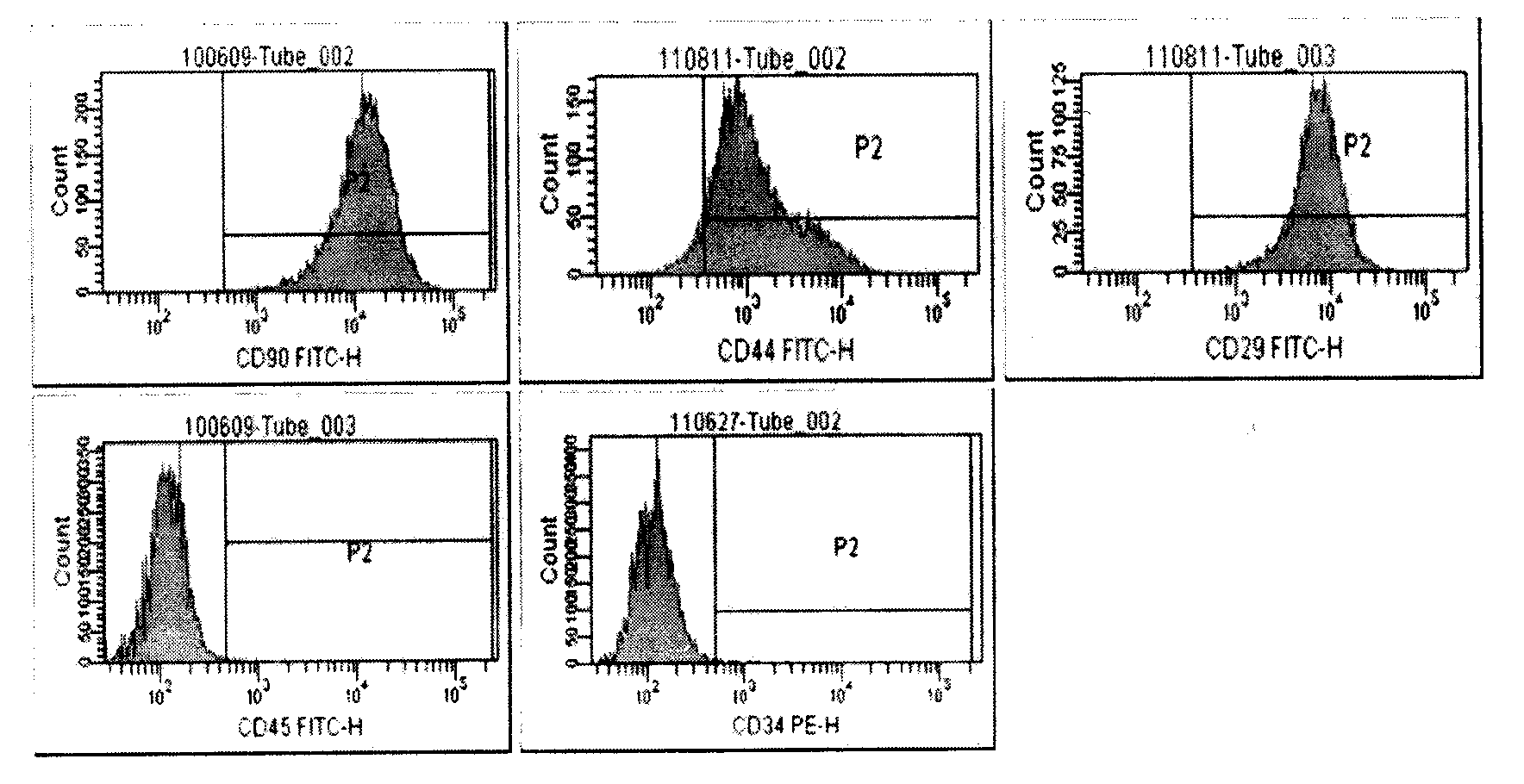
[0063] 1. Identification of BMSCs before use
[0064] (1) Sterility test: If there is no abnormality during the culture process, 3 days before the compounding of BMSCs and collagen scaffolds, after the last liquid change, a sterility test is performed on the supernatant in each culture bottle to culture bacteria and mold.
[0065] (2) Appearance and observation under the microscope: on the day when BMSCs and collagen scaffolds are combined, if the sterility test reports no bacteria and mold growth, observe the color of the supernatant in the culture bottle, and observe the cell morphology and cell morphology under an inverted microscope. Density, presence or absence of bacterial or mold growth. If there is any doubt, another sample must be taken for sterility test to cultivate bacteria and mold.
[0066] (3) Proportion of live cells: On the day when BMSCs and collag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com