Chemical dissociation of cell aggregates
a cell aggregate and chemical technology, applied in the field of chemical dissociation of cell aggregates, can solve the problems of increasing the risk of cell death, affecting the viability of the aggregate, so as to reduce the damage and increase the viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
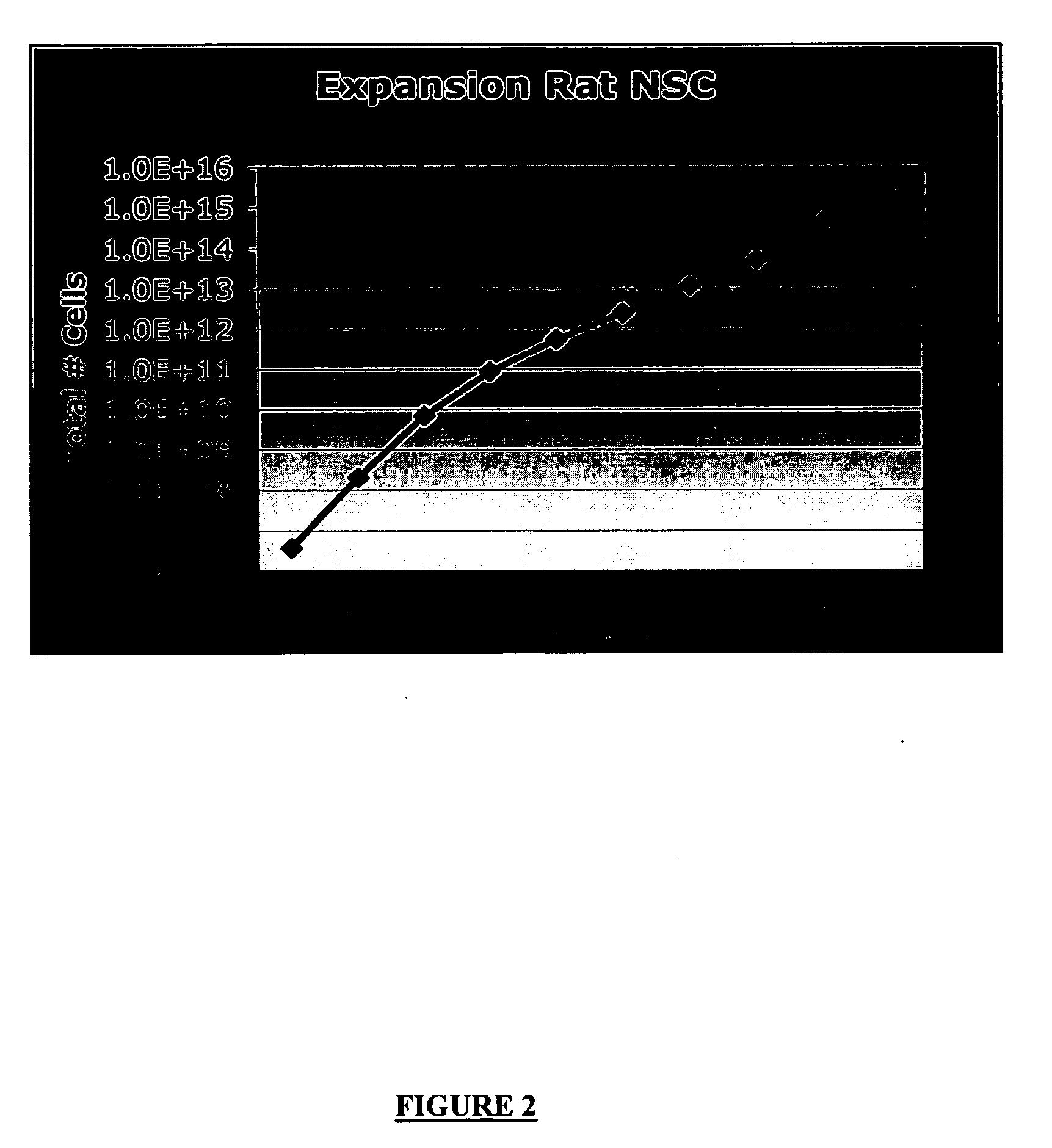
Passaging of Embryonic Mouse Neurospheres
[0136] Neural cells can be obtained from primary embryonic, post-natal or adult CNS tissue from any region of the neuroaxis including but not limited to the striatum, septum, cortex, ventral mesencephalon, midbrain, cerebellum or spinal cord from murine, rodent and human. Neural cells can also be obtained from cultured cells such as those generated using the Neurosphere Assay or any method known to one skilled in the art of neural tissue culture. Neural cells can also be obtained from any stage of embryonic stem cell cultures according to any standard procedure for culturing ES cells.
[0137] For example, striata and / or cortex were dissected from Embryonic Day 14 CD1 albino mouse embryos (Charles River) using standard microdissection techniques. Tissue was collected in phosphate-buffered saline with 2% glucose then mechanically dissociated using a fire-polished glass pipette into a single cell suspension, washed once and resuspended in compl...
example 2
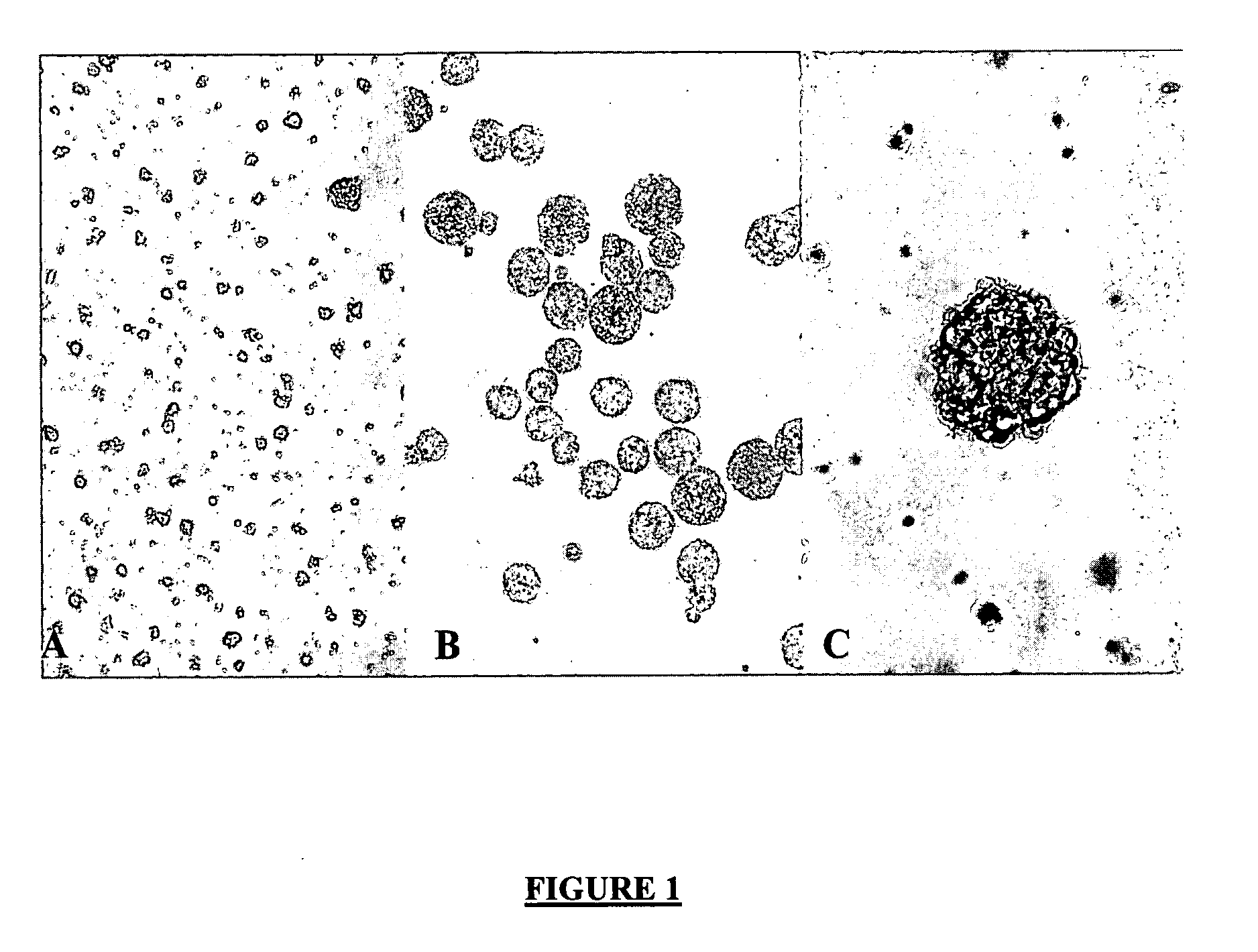
Effect of pH on Aggregate Morphology on Mouse Neurospheres.
[0138] To determine if pH has an affect on the NSC aggregate morphology, several samples of aggregates from 4-day old T-flask cultures were isolated, subjected to a range of environmental pH values, and simply observed over time. Media with different pH values were generated by adding either 1.0 N HCl or 1.0 N NaOH to preferably PPRF-m4 medium or a neural stem cell proliferation medium comprised of a Basal Medium containing, DMEM / F12, glucose, HEPES and sodium bicarbonate; and a proliferation supplement consisting of Insulin, Apo-transferin, Progesterone, Putrescine, Sodium Selenite (O'Connor et al., 1996). Observations revealed that during the time course of the experiment, placing the aggregates in an acidic environment did not result in any significant degree of dissociation. Even after 30 minutes at a pH of 4, the aggregates remained intact, although the cells on the surface of the aggregate appeared rounder and more v...
example 3
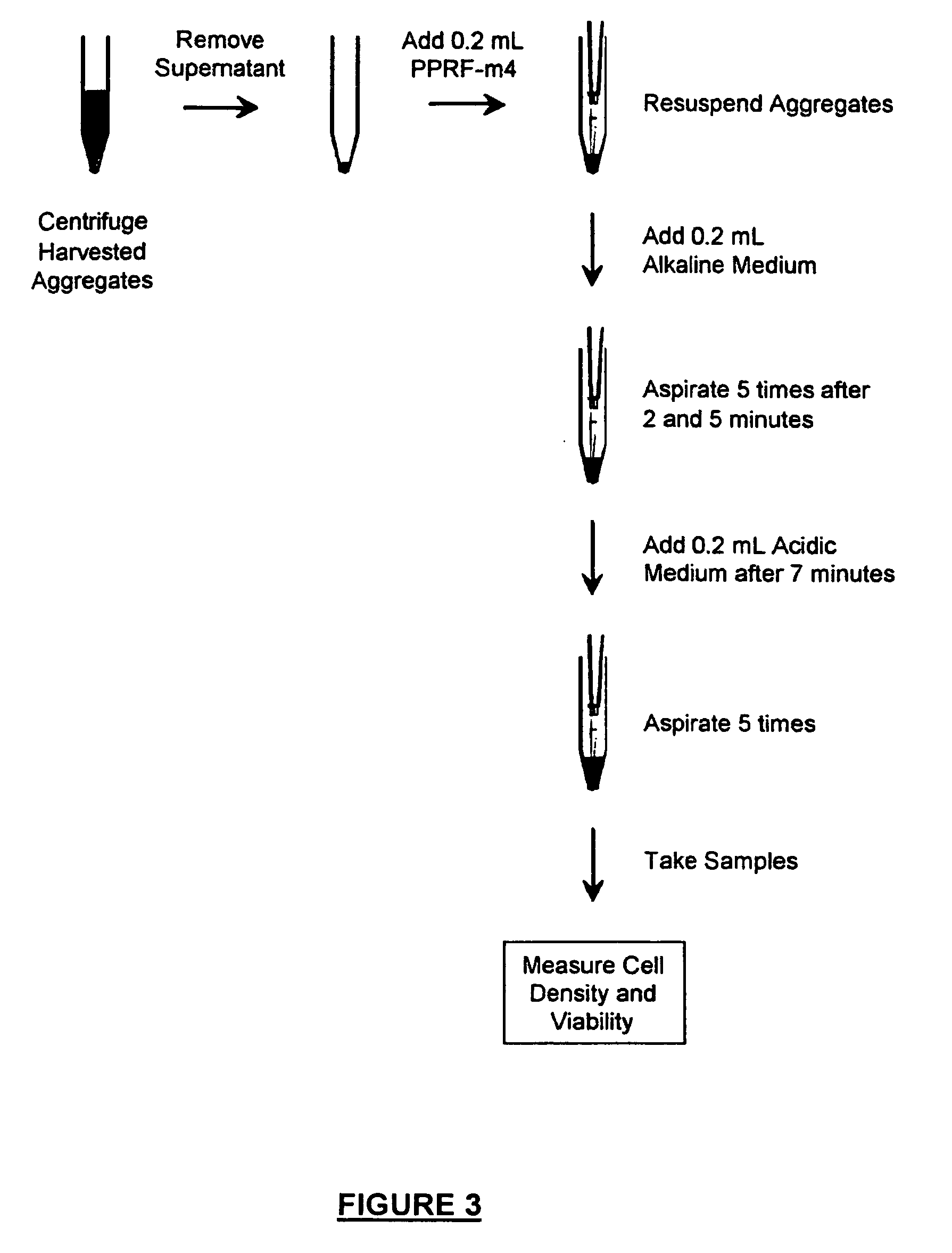
Method of Dissociation of Neurospheres
[0141] Using the above solutions, a 2-step protocol was developed to dissociate a population of NSC aggregates into a single cell suspension. This is schematically depicted in FIG. 3. Briefly, harvested aggregates were centrifuged (10 minutes, 140 g) to form a cell pellet in a 15 mL centrifuge tube. The supernatant was completely removed. The aggregates were then resuspended in 200 μL of fresh PPRF-m4 medium at room temperature by pipetting the cell pellet 5 times. 200 μL of the alkaline medium was then added to the tube, and a stopwatch was used to time the procedure for 7 minutes. After 2 minutes and 5 minutes had elapsed, the cells were gently pipetted 5 times. After 7 minutes had elapsed, 200 μL of the acidic medium was added to the mixture to decrease the pH, and the sample was gently pipetted 5 more times. This procedure reliably resulted in a single cell suspension. This novel chemical dissociation procedure is efficient, cost effective...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com