Use of repeat sequence protein polymers in personal care compositions
a repeat sequence protein and composition technology, applied in the field of personal care compositions, can solve the problems of reducing the affinity of the protein for the negatively charged skin and hair, forming tight, hard fibers that are not suitable for film formation, and not showing all desired characteristics when used in personal care products, so as to improve skin tone and increase the viability of skin fibroblasts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of SELP
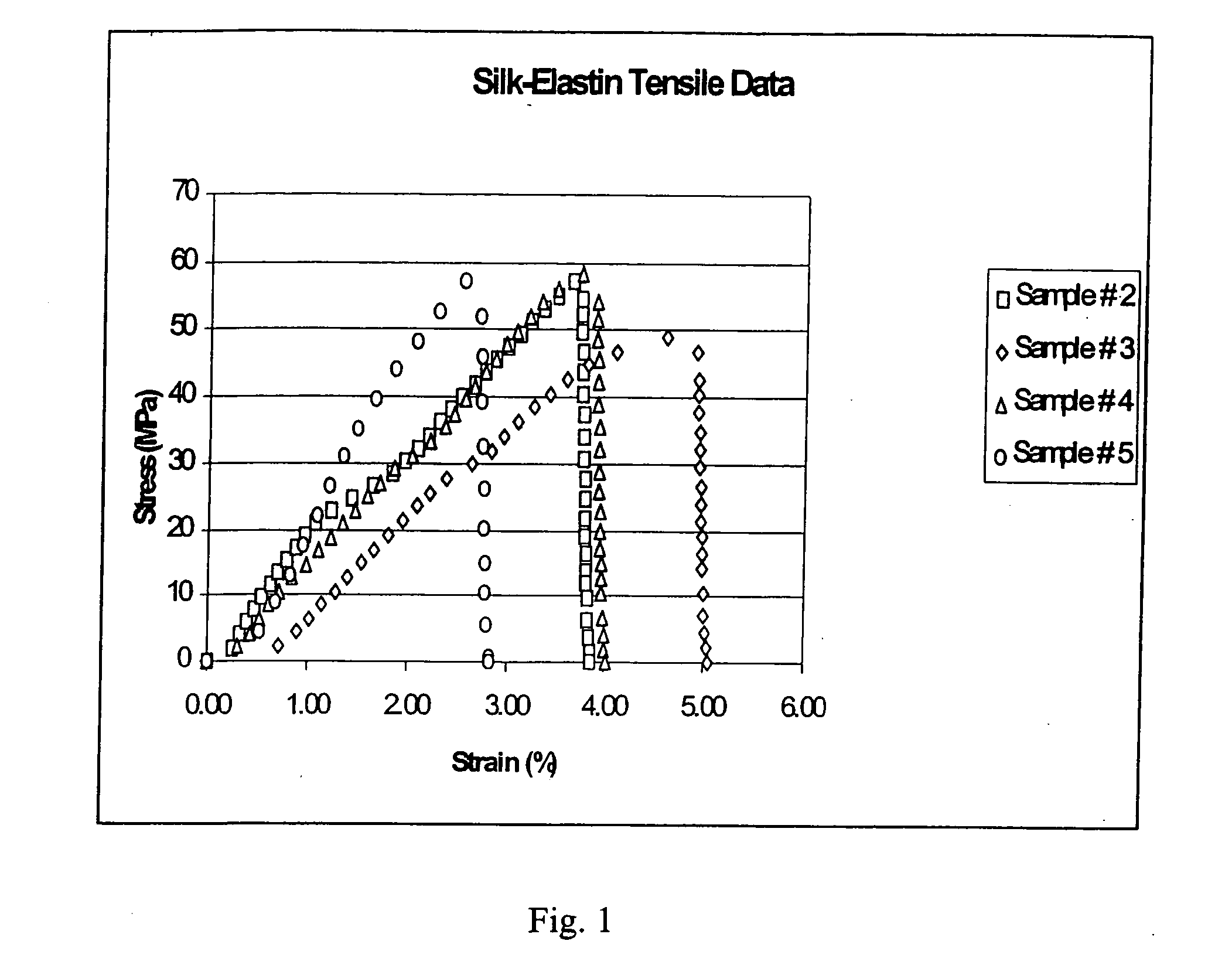
[0167] In this Example, experiments conducted to isolate and purify a genetically engineered silk-elastin repeat sequence protein block copolymer (SELP) from E. coli are described. E. coli containing a specific silk-elastin repeat sequence protein copolymer SELP47K (GC2596-26-A) recombinant DNA was obtained from PPTI. The E. coli may be prepared in accordance with the methods described in U.S. Pat. Nos. 5,243,038 and 6,355,776, incorporated herein by reference. The recovery of kilogram quantities of SELP was also demonstrated. The silk-elastin copolymer SELP47K had a general structure of head—[(GAGAGS)2(GVGVP)3GKGVP(GVGP)4(GAGAGS)2]13-tail (SEQ ID NO:19). The copolymer contained 886 amino acids, with 780 amino acids in the repeating sequence unit. The SELP47K had a molecular weight of about 70,000 Daltons, and the pI of the protein is 10.5.
[0168] Monodispersed silk-elastin protein polymer SELP47K was produced for application testing in the following manner. E. col...
example 2
Preparation of Polydispersed SELP47K for Application Testing
[0181] Experiments involving the purification and preparation of the polydispersed SELP47K silk-elastin protein polymer (GC2596-26-B) for application testing are described in this Example. Cell separation from the fermentation broth was done using microfiltration. A cell disruption to make a cell-extract was done using a French-press. The cell extract was separated from the cell-debris using polyethyleneimine and a filter-aid. The cell extract was mixed with ammonium sulfate to 25% saturation to precipitate the silk-elastin protein polymer. The precipitated silk-elastin protein polymer was further purified by dissolving it in water and reprecipitating it with ammonium sulfate.
[0182] In order to prepare a polydispersed silk-elastin protein polymer, the precipitated silk-elastin protein polymer was again dissolved in water and mixed with a trace amount of protease (BPN′Y217L) (Genencor International). The protease was then ...
example 3
Cleavage of Monodispersed SELP47K
[0184] This Example describes the methods used to cleave monodispersed SELP47K to its monomeric unit. The purification and formation of monomeric unit of SELP47K (4920 kDA molecular weight) (GC2596-26-C) was carried out using monodispersed material of SELP47K produced as in Example 1. The monodispersed SELP47K was dissolved in water and was treated with endopeptidase lysC protease (Sigma) specific for cleaving protein at lysine residue for 30 minutes at room temperature. The lysC protease was then inactivated and destroyed by acid treatment. The monomeric unit of SELP47K was then ultra-filtered until protein polymer solution conductivity reached 50 μS / m2. The monomeric unit of SELP47K solution was then lyophilized to get the solid material and stored at −70 C prior to application testing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight % | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com