Separating and culturing process of human amnion mesenchyme stem cell and its medical composition
A technology of stromal stem cells and culturing methods, applied in the field of separation and culturing of mesenchymal stem cells, can solve the problems of limited number of stem cells due to ethical restrictions, and achieve the effect of being free from ethical restrictions, having a wide range of sources and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
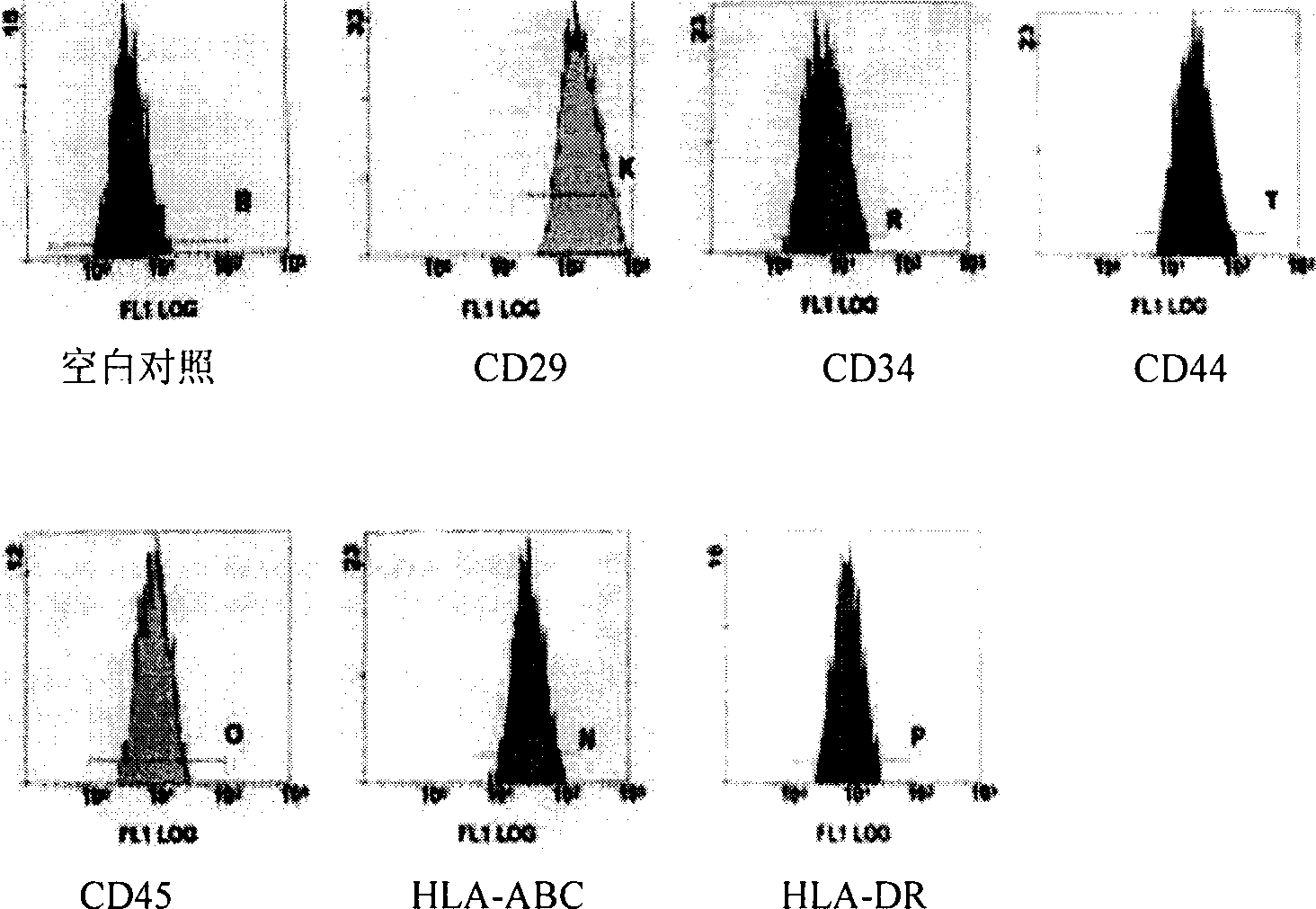
[0019] Example 1: Isolation, culture, expansion and purification of human amniotic mesenchymal stem cells
[0020] 1. Isolation of human amniotic mesenchymal cells:
[0021] Under sterile conditions, human placenta from normal full-term caesarean section fetuses was taken, and the amniotic membrane on the umbilical cord surface of the placenta was bluntly separated, washed with phosphate buffered solution (PBS), and the amniotic membrane was cut into pieces with a size of 1.0cm×1.0cm. Add 2 ml of 0.25% trypsin to 1 gram of tissue, digest at room temperature for 30 minutes, 3 times in total, then stop trypsin with DMEM / F12 culture medium containing 10% fetal bovine serum (FBS) by volume, To remove as much epithelial cells as possible. Then the amniotic membrane was cut into pieces as much as possible, and V containing 1.0g / L collagenase and 0.10g / L deoxyribonuclease was added per gram of tissue. DMEM :V F12 = 2ml of 1:1 DMEM / F12 culture solution, digested at 37°C for 1 hour ...
Embodiment 2
[0028] Example 2: Directional induction of HADMSCs to differentiate into neuron-like cells
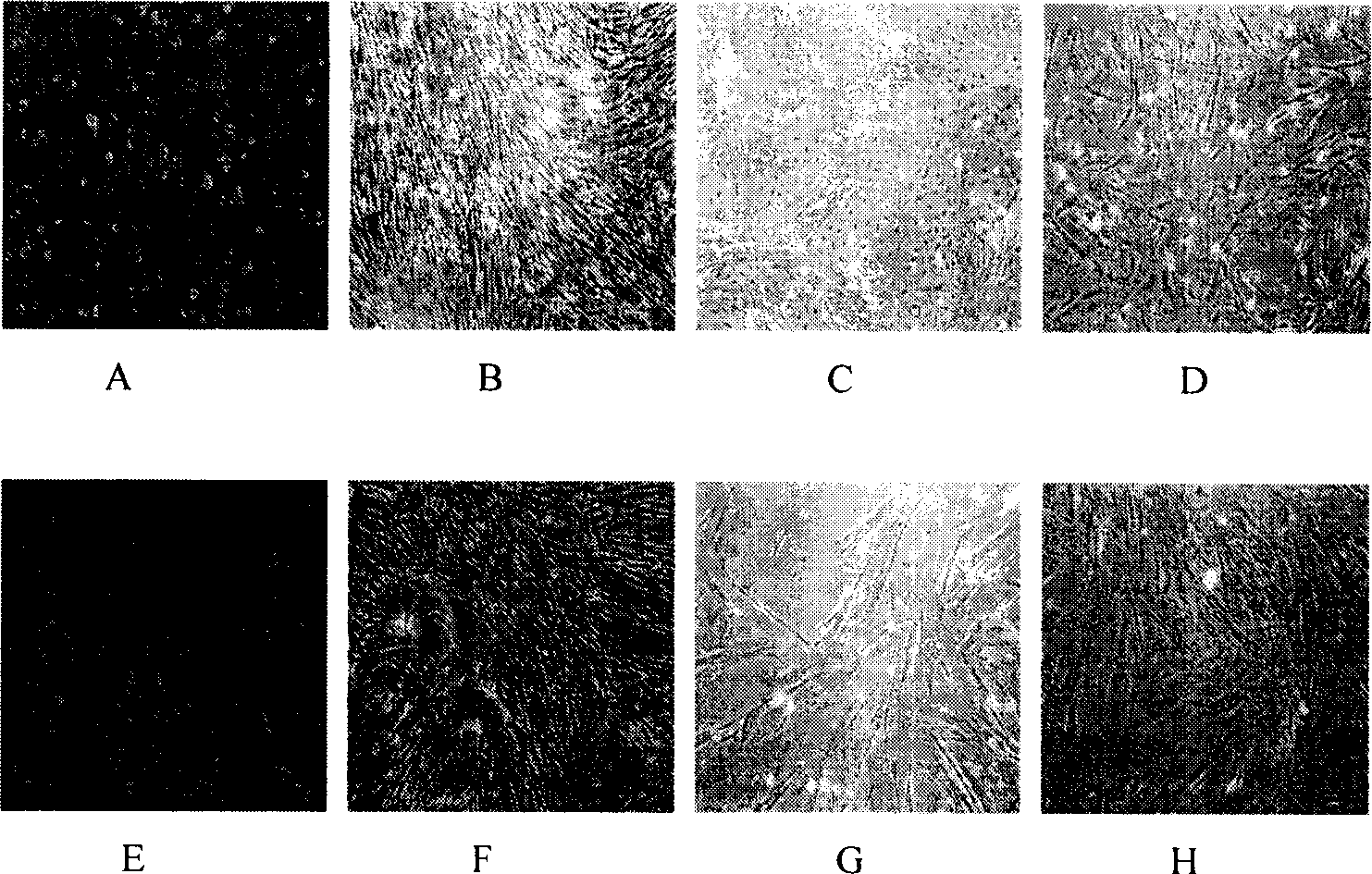
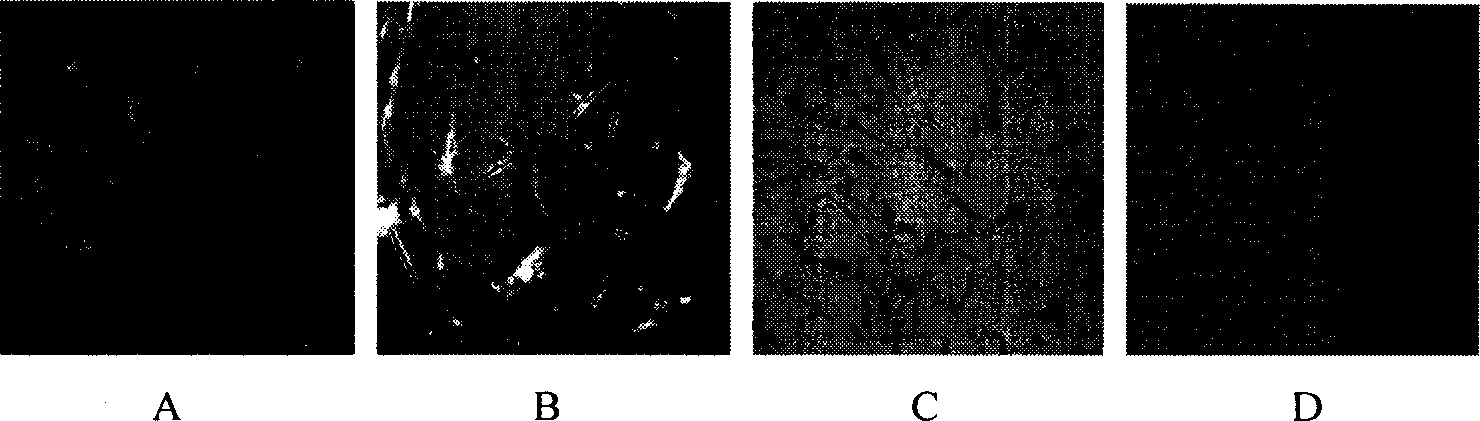
[0029] 1. Orientation induction: take the HADMSCs passed to the third generation and press 4×10 5 / L density was seeded in a six-well plate with sterilized coverslips placed in advance to prepare cell slides. When the cells reached 80% confluence, the cells were mixed with 30 μmol / L all-trans retinoic acid (Retinic acid, purchased from sigma company), DMEM / F12 medium with 20ng / mlbFGF and 10% FBS was induced for 7 days, and no inducer was added to the control group. Results: The cultured human amniotic mesenchymal stem cells expressed neural stem cell marker human nestin (nestin), and the induced cells expressed nestin and neuron marker human neuron-specific enolase (NSE). Expression of glial cell marker glial fibrillary acidic protein (GFAP).
[0030] 2. Observation under an inverted microscope: HADMSCs are induced to differentiate into nerve cells. Two days after adding the inductio...
Embodiment 3
[0033] Example 3: Application of the composition containing HADMSCs in the treatment of nervous system diseases
[0034] HADMSCs can differentiate into nerve cells, opening up a new cell source for nerve transplantation. HADMSCs can be transplanted into human body through local application or lumbar puncture and intravenous infusion to treat neurological diseases such as brain injury, Parkinson's syndrome, stroke, spinal cord injury and peripheral nerve injury.
[0035] 1. Animal grouping and model making:
[0036] 80 Wistar rats, male or female, were randomly divided into 4 groups: Group A was the injury group (Sham), 20 rats, with only craniotomy and drilling without hitting brain tissue and transplanting cells, as a negative control group. Group B is the sham transplantation group (TBI+NS), 20 rats were injected with 10 μl of normal saline 1 day after craniotomy and drilling, as an intervention control. Group C is the transplantation group (TBI+HADMSCs), 20 rats were inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com