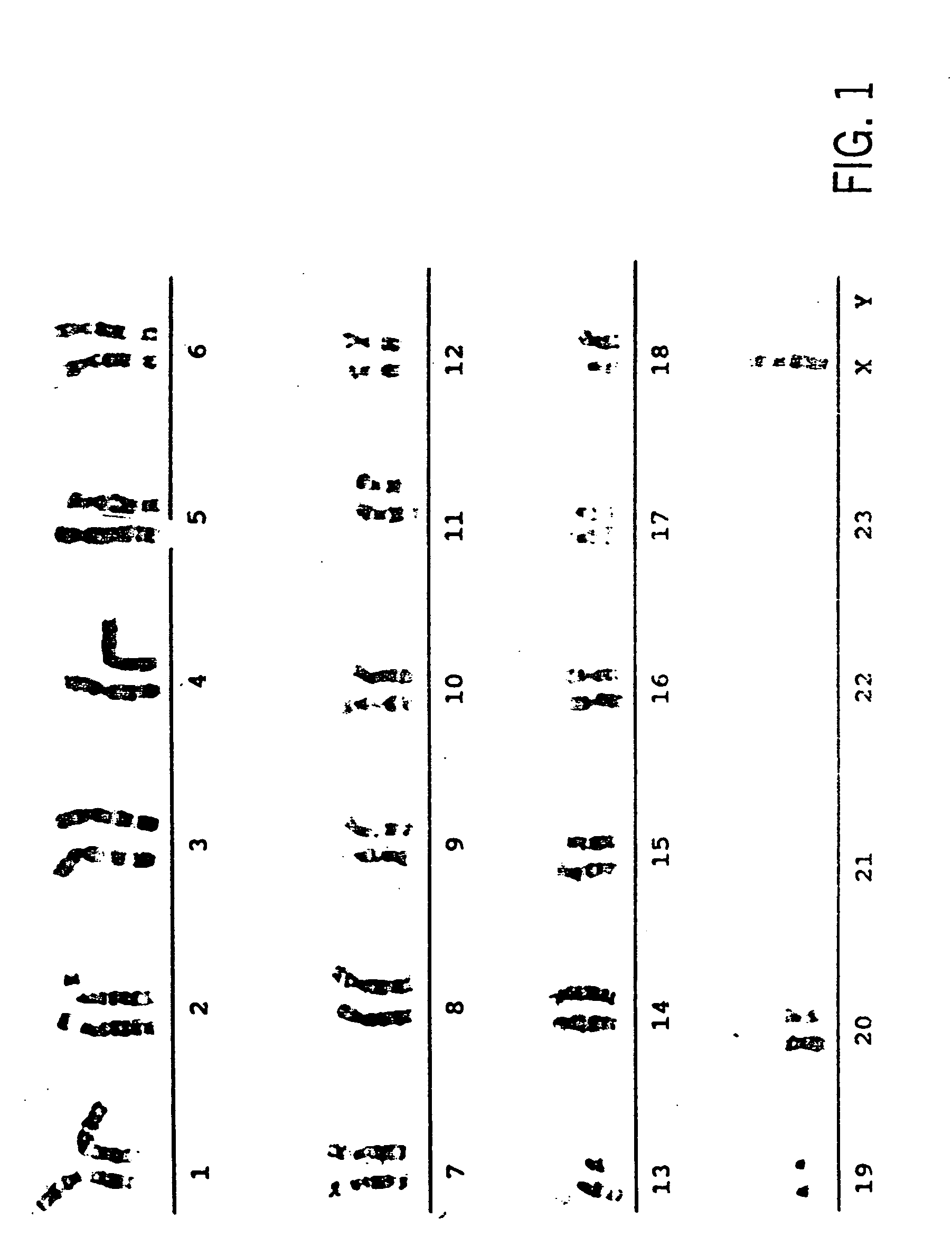
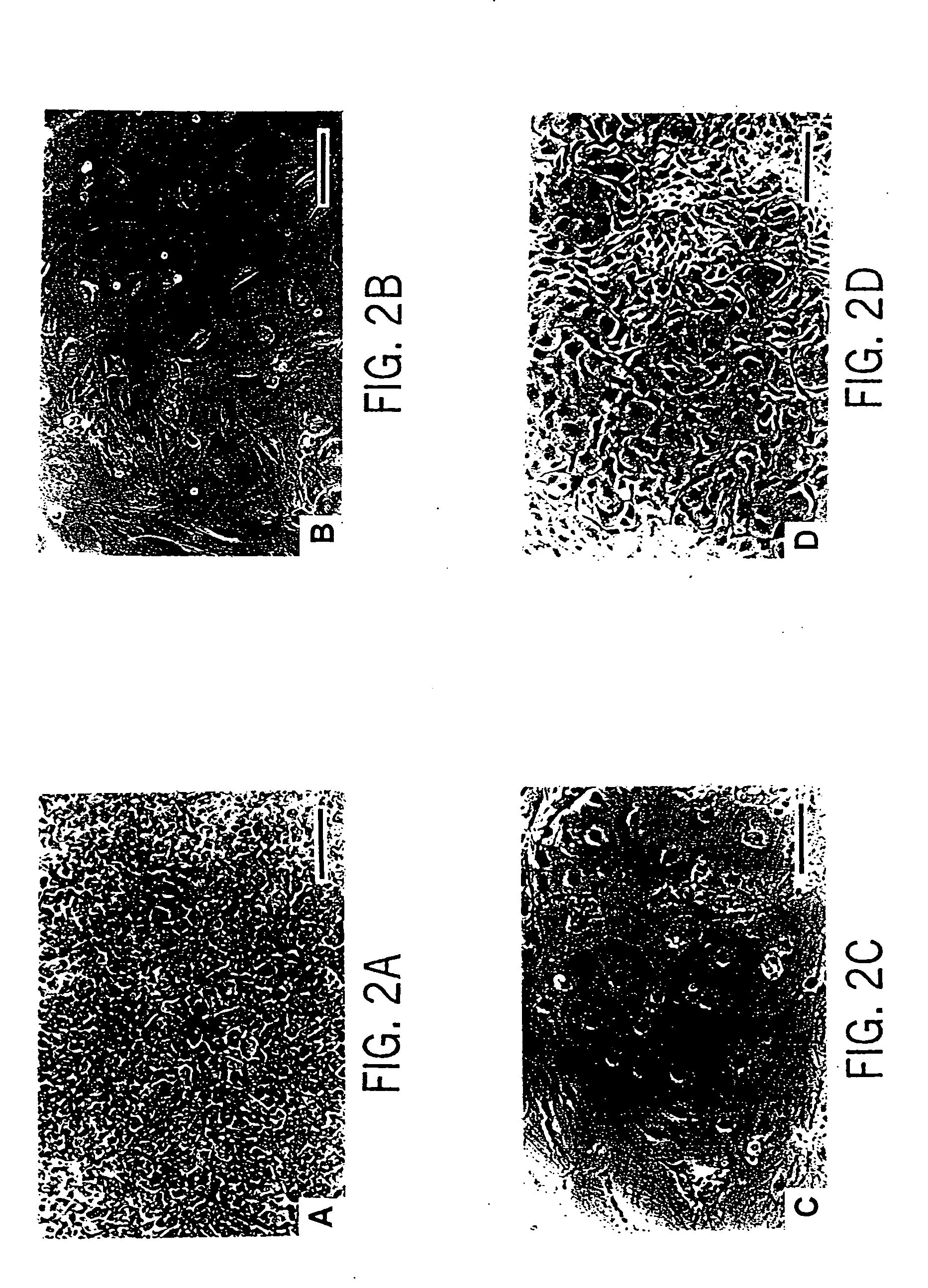
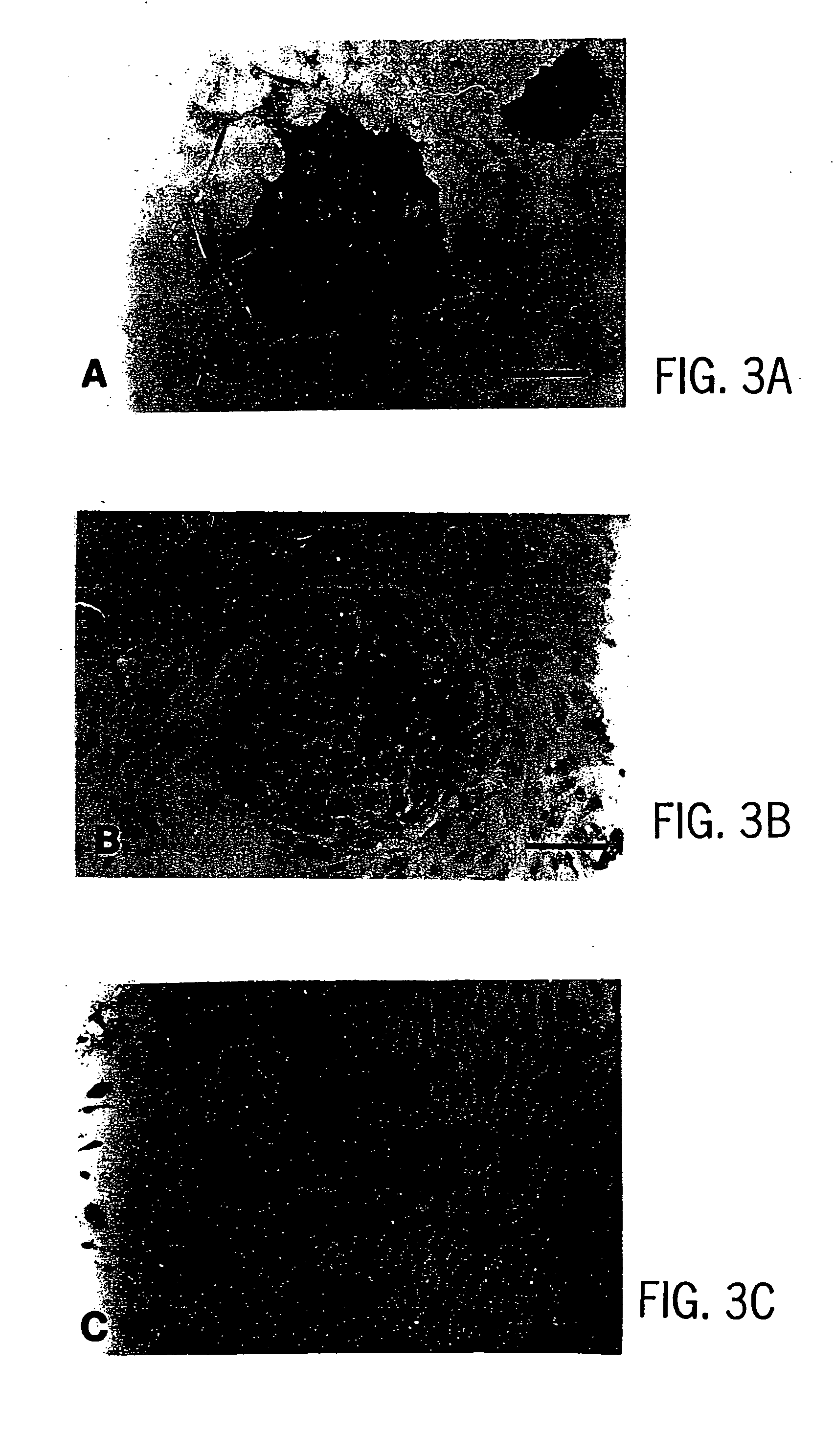
Primate embryonic stem cells
a technology of stem cells and embryos, applied in the field of stem cell cultures, can solve the problems of limited range of differentiation of human ec cells, pregnancy failure, and limited usefulness of mouse es cells as a model of human development, and achieve the effect of high nucleus/cytoplasm ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
(1) Animals and Embryos
[0077] As described above, we have developed a technique for non-surgical, uterine-stage embryo recovery from the rhesus macaque and the common marmoset.
[0078] To supply rhesus embryos to interested investigators, The Wisconsin Regional Primate Research Center (WRPRC) provides a preimplantation embryo recovery service for the rhesus monkey, using the non-surgical flush procedure described above. During 1994, 151 uterine flushes were attempted from rhesus monkeys, yielding 80 viable embryos (0.53 embryos per flush attempt).
[0079] By synchronizing the reproductive cycles of several marmosets, significant numbers of in vivo produced, age-matched, preimplantation primate embryos were studied in controlled experiments for the first time. Using marmosets from the self-sustaining colony (250 animals) of the Wisconsin Regional Primate Research Center (WRPRC), we recovered 54 viable morulae or blastocysts, 7 unfertilized oocytes or degenerate embryos, and 5 empty z...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com