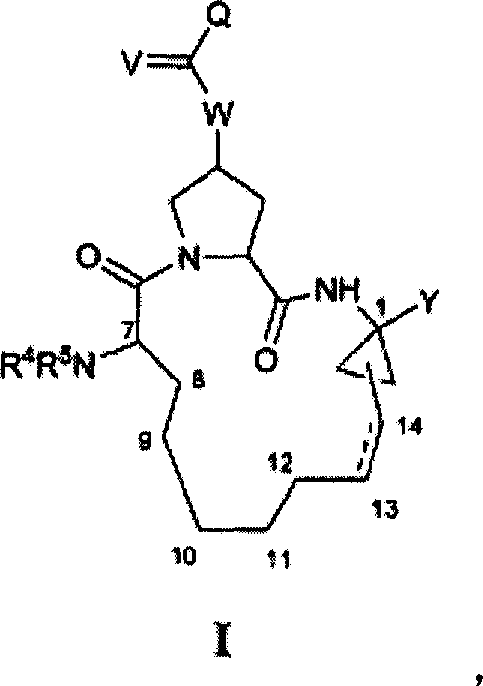
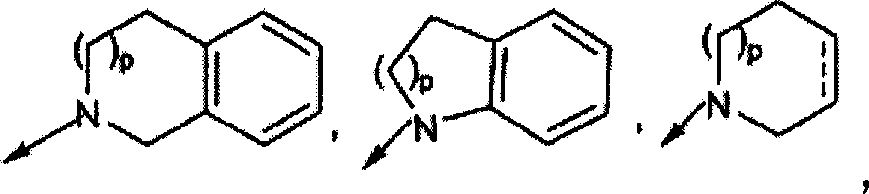
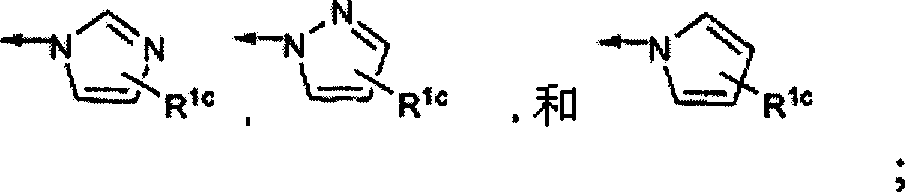Macrocyclic compounds as inhibitors of viral replication
A compound, cycloalkyl technology, used in antiviral agents, drug combinations, organic chemistry, etc., to solve problems such as non-response, patients without treatment options, and patient treatment failures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1020] Preparation of Part A Virus Inhibitors
[1021] Compounds of general formula I can be synthesized in the same general manner as described below for compounds of general formula II-XIX. The synthesis of various specific compounds of general formula I is described in the examples below. Variations in sequence will be appreciated by those skilled in the art and will further appreciate the importance of appropriate reaction conditions for similar reactions shown or otherwise known that may be suitably used in the processes described hereinafter for the preparation of compounds of formula I. Variety.
[1022] The products of the reactions described herein are isolated by conventional means such as extraction, distillation, chromatography and the like.
[1023] Salts of compounds of the above formulas are prepared by reacting an appropriate base or acid with a stoichiometric equivalent of a compound of formula I.
[1024] Preparation of Part B Virus Inhibitors
[1025...
example 1-1
[1030] Example 1-1: Synthesis of Compound #101 (Compound AR00220042) by Method A:
[1031]
[1032] Compound #101 (Compound AR00220042)
[1033] Method A:
[1034]
[1035] Step 1: Synthesis of tert-butyl 2S-(1-ethoxycarbonyl-2-vinyl-cyclopropylcarbamoyl)-4R-hydroxy-pyrrolidine-1-carboxylate (3)
[1036]
[1037] Add ethyl-(1R, 2S) / (1S, 2R)-1-amino-2-vinylcyclopropylcarboxylate (1, 1.0g, 5.2mmol), trans-N-(tert-butyl A solution was made by adding 30 mL of DMF to a flask of oxycarbonyl)-4-hydroxy-L-proline (2, 1.3 g, 1.1 eq) and HATU (2.7 g, 1.1 eq). The solution was cooled to 0°C in an ice-water bath, then a solution of DIEA (4.4ml, 4eq) in DMF (15ml) was added slowly with stirring. The reaction was allowed to warm to room temperature and stirred overnight.
[1038] After 16 h, the reaction was monitored for completion by HPLC. Diluted with EtOAc (100 mL), water (3×40 mL), saturated sodium bicarbonate (NaHCO 3 (2×40mL)) and brine (2×40mL), followed by Na 2 ...
example 1-2
[1059] Example 1-2: Synthesis of Compound #101 (Compound AR00220042) by Method B:
[1060] Method B:
[1061]
[1062] Compound #101 was also prepared according to the above procedure. The synthesis of macrocyclic intermediate 10 described herein is analogous to that described in International Application PCT / CA00 / 00353 (publication No. WO 00 / 59929).
[1063] Step 1: Synthesis of tert-butyl 2S-(1-ethoxycarbonyl-2-vinyl-cyclopropylcarbamoyl)-4R-hydroxy-pyrrolidine-1-carboxylate (3)
[1064]
[1065] Add ethyl-(1R, 2S) / (1S, 2R)-1-amino-2-vinylcyclopropylcarboxylate (1, 1.0g, 5.2mmol), trans-N-(tert-butyl A solution was made by adding 30 mL of DMF to a flask of oxycarbonyl)-4-hydroxy-L-proline (2, 1.3 g, 1.1 eq) and HATU (2.7 g, 1.1 eq). The solution was cooled to 0°C in an ice-water bath, then a solution of DIEA (4.4ml, 4eq) in DMF (15ml) was added slowly with stirring. The reaction was allowed to warm to room temperature and stirred overnight.
[1066] After 16 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com