Integrin antagonists with enhanced antibody dependent cell-mediated cytoxicity activity
a technology of cytoxicity activity and integrin antagonist, which is applied in the direction of peptides, drug compositions, and infusion cells, can solve the problems of pathological conditions, increased destruction, and ineffective current treatment options, such as surgery, chemotherapy and radiation treatment, and achieves enhanced and reduced adcc and/or cdc activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
7.1 Example 1
Construction and Expression of Novel Fc Variants of Antibodies
[0278] Based on the structural information available for the Fc-FcγRIIIB complex, each of the putative FcγR contact residues of the IgG1 Fc portion was randomly mutated by using degenerated oligonucleotides incorporating all possible single mutations. The contact residues were divided into four regions (RI: Leu234, Leu235, Gly236, Gly237, Pro238, Ser239; RII: Asp265, Ser267, Glu269; RIII: Ser298; and RIV: Ala327, Leu328, Pro329, Ala330, and Ile332). Primers used for the amplification and library construction are listed in table 4. The IgG1 of antibody Vitaxin™, converted into scFv-Fc format, was used as the model for this study. The DNA and corresponding amino acid sequences of the variable regions of the Vitaxin® heavy and light chains used to generate the scFv-Fc are shown in FIG. 1 (panels A and B, respectively). The scFv-Fc was then harnessed as the template to build three Fc mutant libraries containing...
example 2
7.2 Example 2
Construction and Expression of the Extracellular Domains of FcγRIIIA and FcγRIIB
[0284] To facilitate the binding studies of the Fc variants to FcγRs the extracellular domains of FcγRIIIA and FcγRIIB were subcloned for expression as strepavidin fusion proteins in E. coli and for expression in mammalian cells. The FcγRIIIA prepared for analysis is the low affinity (F158) allotype. Two forms of FcγRIIIA and FcγRIIB were prepared, a “tetramer” form, generated as as Strepavidin fusion, and a “monomer” form generated as a Flag-tagged.
7.2.1 Materials and Methods
[0285] Construction and Bacterial Expression of the Extracellular Domains of FcγRIIIA- and FcγRIIB-Strepavidin Fusion Proteins (Tetramer): Primer pairs SA1 / SA2, A1 / A2, and B1 / B2 (see primer list, Table 4) were used to PCR amplify streptavidin and the extracellular domains of FcγR IIIA and FcγR IIB, respectively. The cDNA library of human bone marrow (Clontech) was used as a template for FcγR IIIA and FcγR IIB ampli...
example 3
7.3 Example 3
Characterization of the Fc Variants
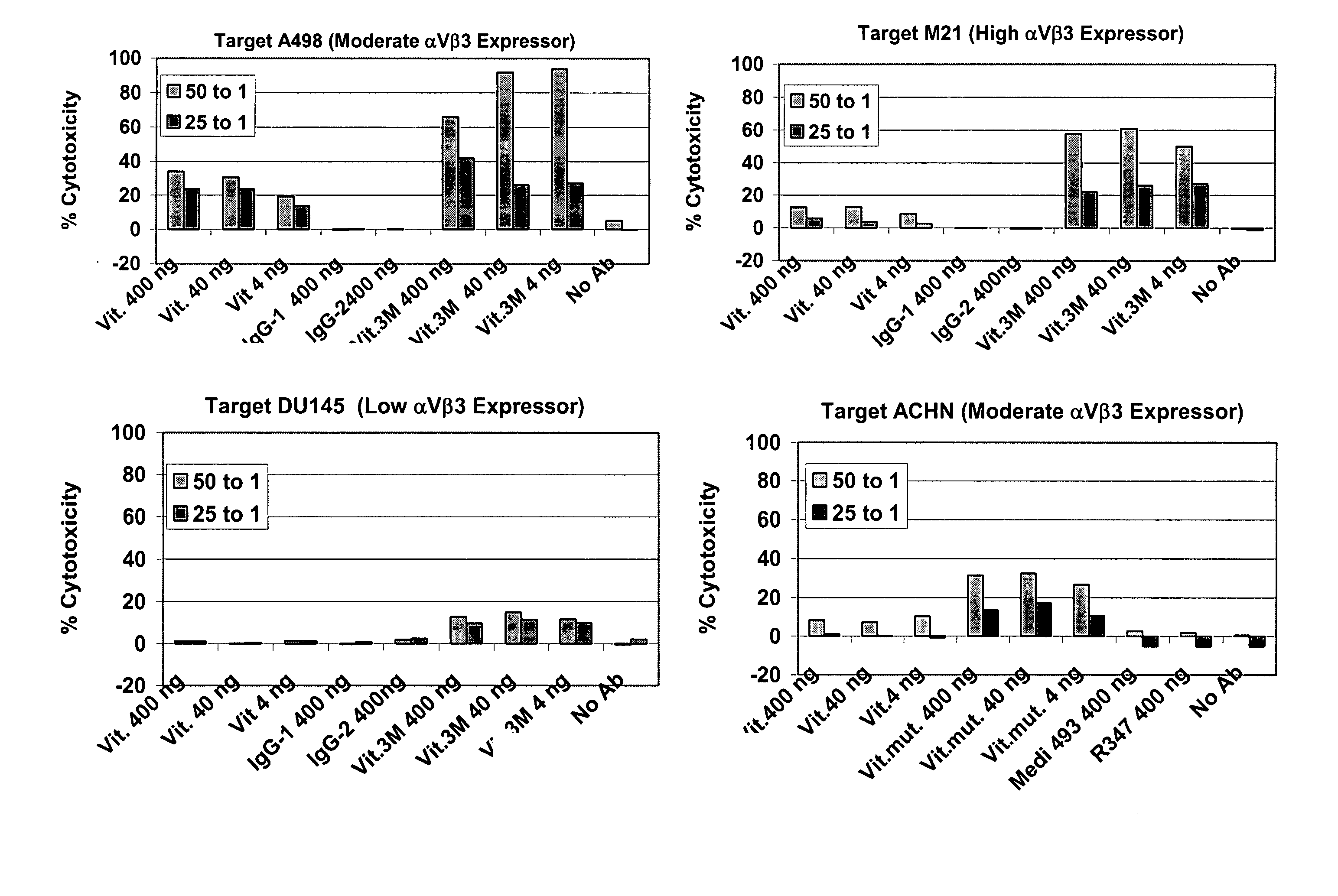
[0287] After mutagenesis of the Fc domain (see example 1 supra) Fc variants, in the scFV-Fc fusion format, were screened for enhanced binding to FcγRIIIA tetramer by ELISA as detailed below. The results for several clones are shown in FIG. 5. In addition, the ADCC activity of these clones was determined against M21 cells. The results for several clones are shown in FIG. 6. Based on these studies three substitutions were chosen for further study, S239D, A330L and I332E. These substitutions were introduced into the Fc region of the intact Vitaxin® IgG1 heavy chain and coexpressed with Vitaxin® light chain to produce full length Vitaxin® Fc variant IgG1 molecules. The Vitaxin® Fc variant having the I332E substitution was designated Vitaxin®-1M, the Vitaxin® Fc variant having the S239D, A330L, I332L triple substitution was designated Vitaxin®-3M.
[0288] A panel of Vitaxin® Fc variants, in IgG format, was generated in which each of the st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com