Recombinant il-9 antibodies and uses thereof
a technology of il-9 antibodies and antibodies, which is applied in the field of recombinant il-9 antibodies, can solve the problems of causing harmful or deadly immunological responses, increasing the number of defenseless patients, swelling, redness, warmth, etc., and achieves the effect of reducing the severity and/or duration of a diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6.1 Example 1
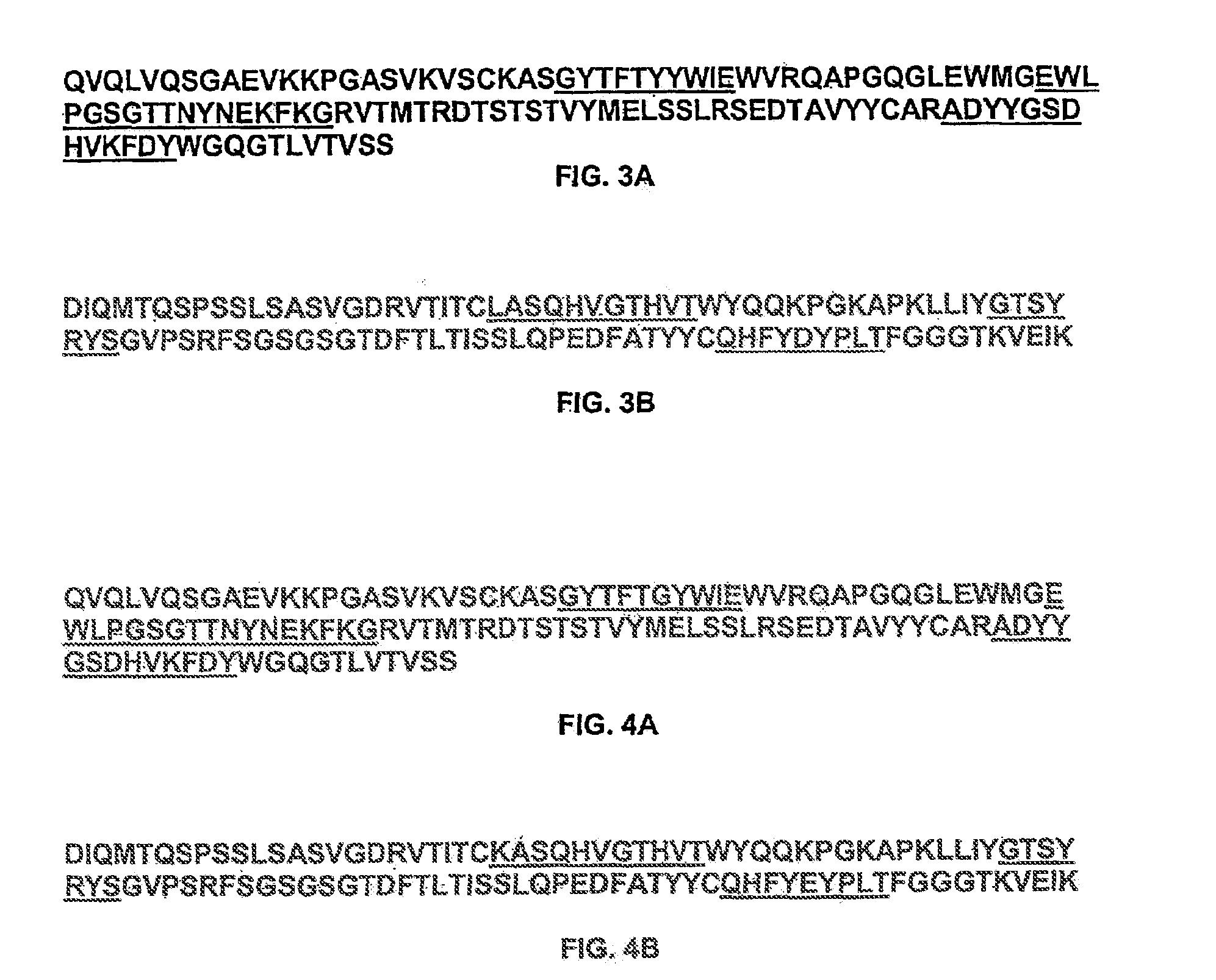
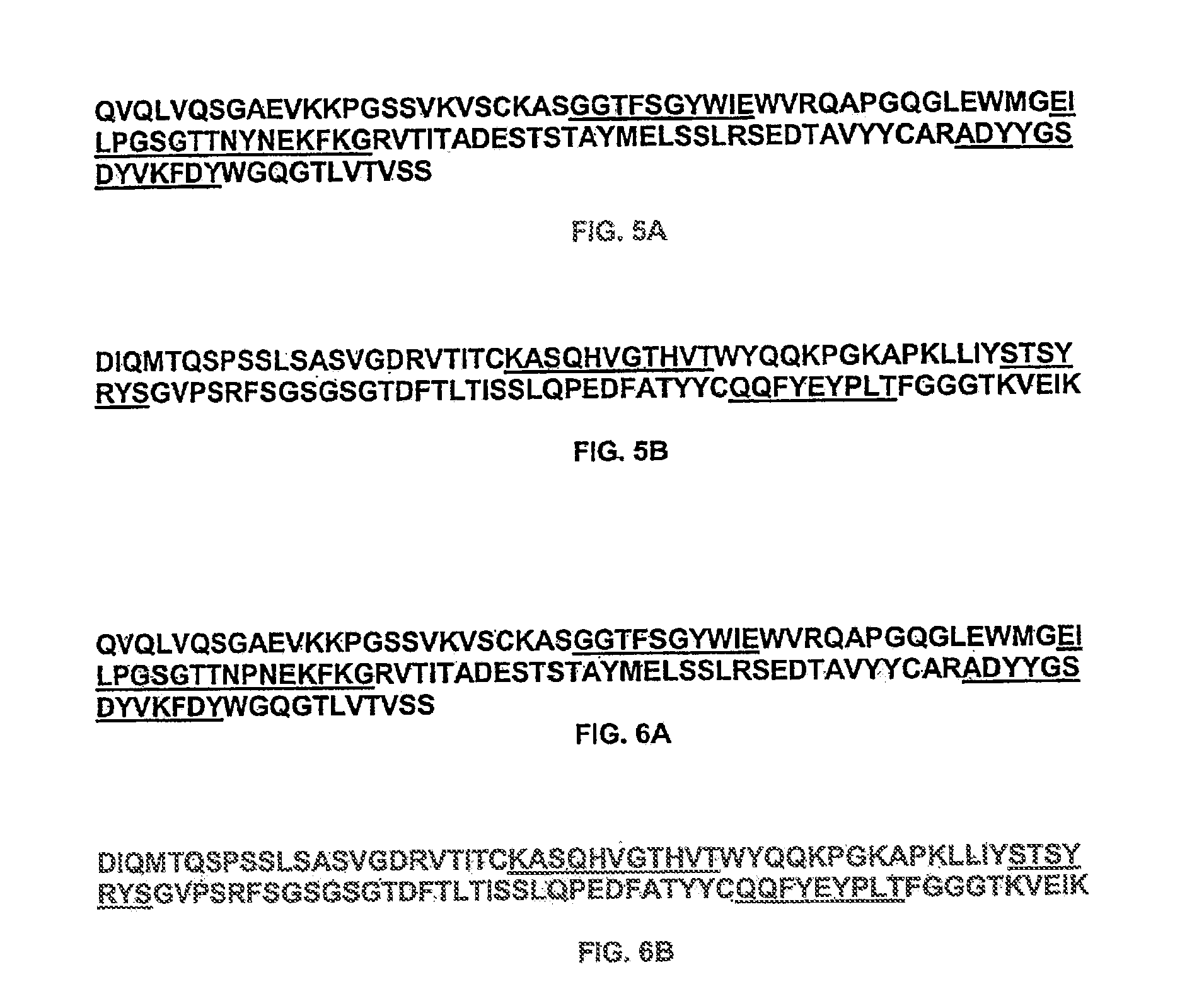
Preparation of Anti-IL-9 Antibody 7F3com-2H2
[0575]The anti-IL-9 humanized monoclonal antibody 7F3com-2H2 was prepared using the pMI347 vector. The pMI347 vector coding for the expression of 7F3com-2H2 consists of the following four independent genetic elements: a glutamine synthetase selectable marker expression cassette, the 2H2 kappa light chain cDNA expression cassette, the 2H2 γ1 heavy chain mini-gene expression cassette, and a bacterial origin of replication and antibiotic resistance gene.
[0576]The first element, the glutamine synthetase selectable marker expression cassette consists of hamster glutamine synthetase (GS) cDNA under the control of simian virus 40 (SV40) early enhancer and promoter and SV40 early splicing and polyadenylation region for efficient mRNA cleavage and addition of a polyadneylate tail. This element is required for integration, amplification, and stable maintenance of the plasmid in the host cell genome.
[0577]Each of the 2H2 heavy and light ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com