Pharmaceutical composition containing oleanolic acid acetate as an active ingredient for preventing or treating TLR- or IL-6-mediated diseases
A technology of IL-6 and active ingredients, applied to skin external preparations for good TLR and IL-6-mediated diseases, relates to the field of prevention or health-care functional foods containing the active ingredients, and can solve pathological and The genetic mechanism of action has not been clearly elucidated, etc., to achieve the effect without side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
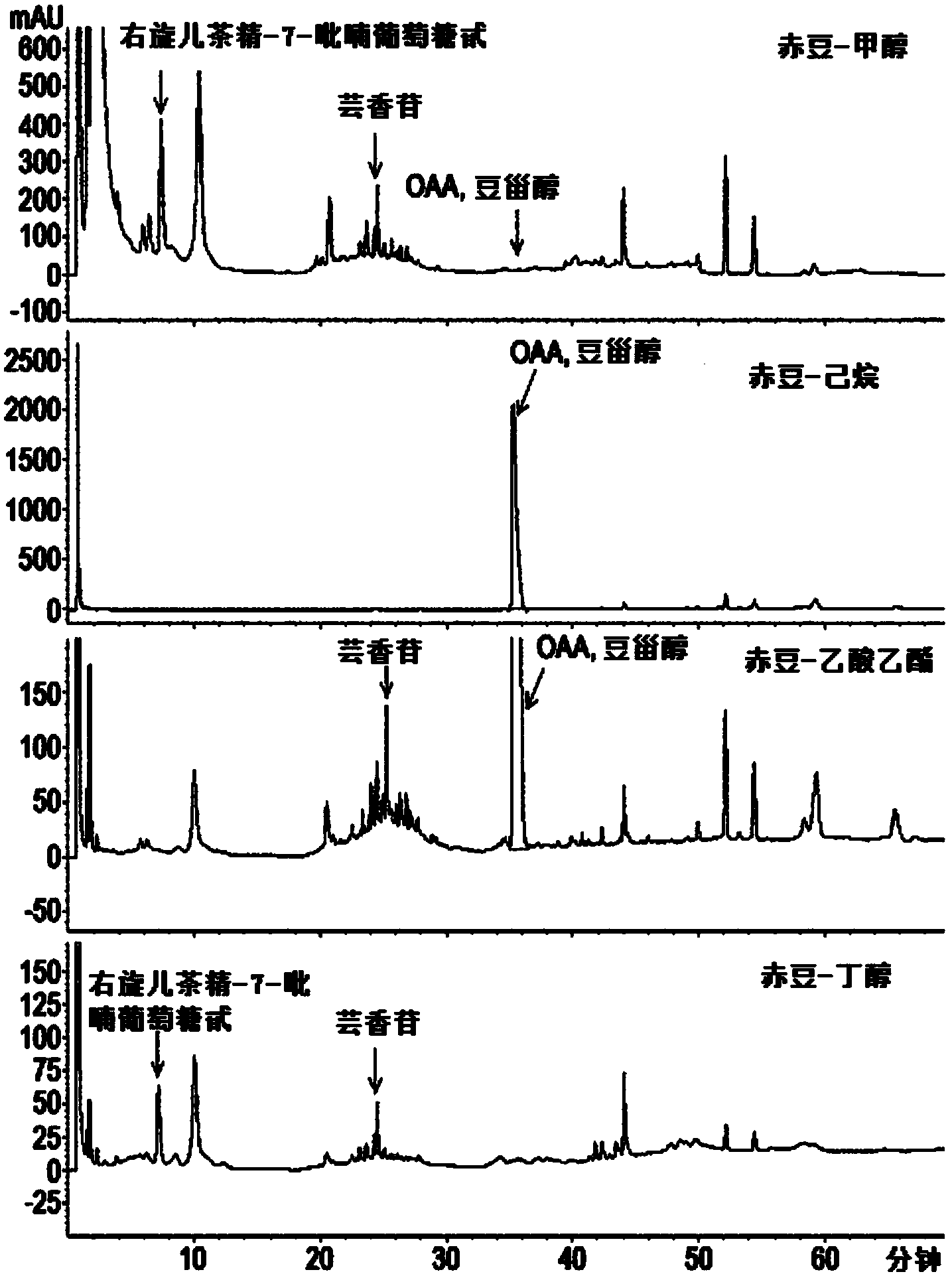
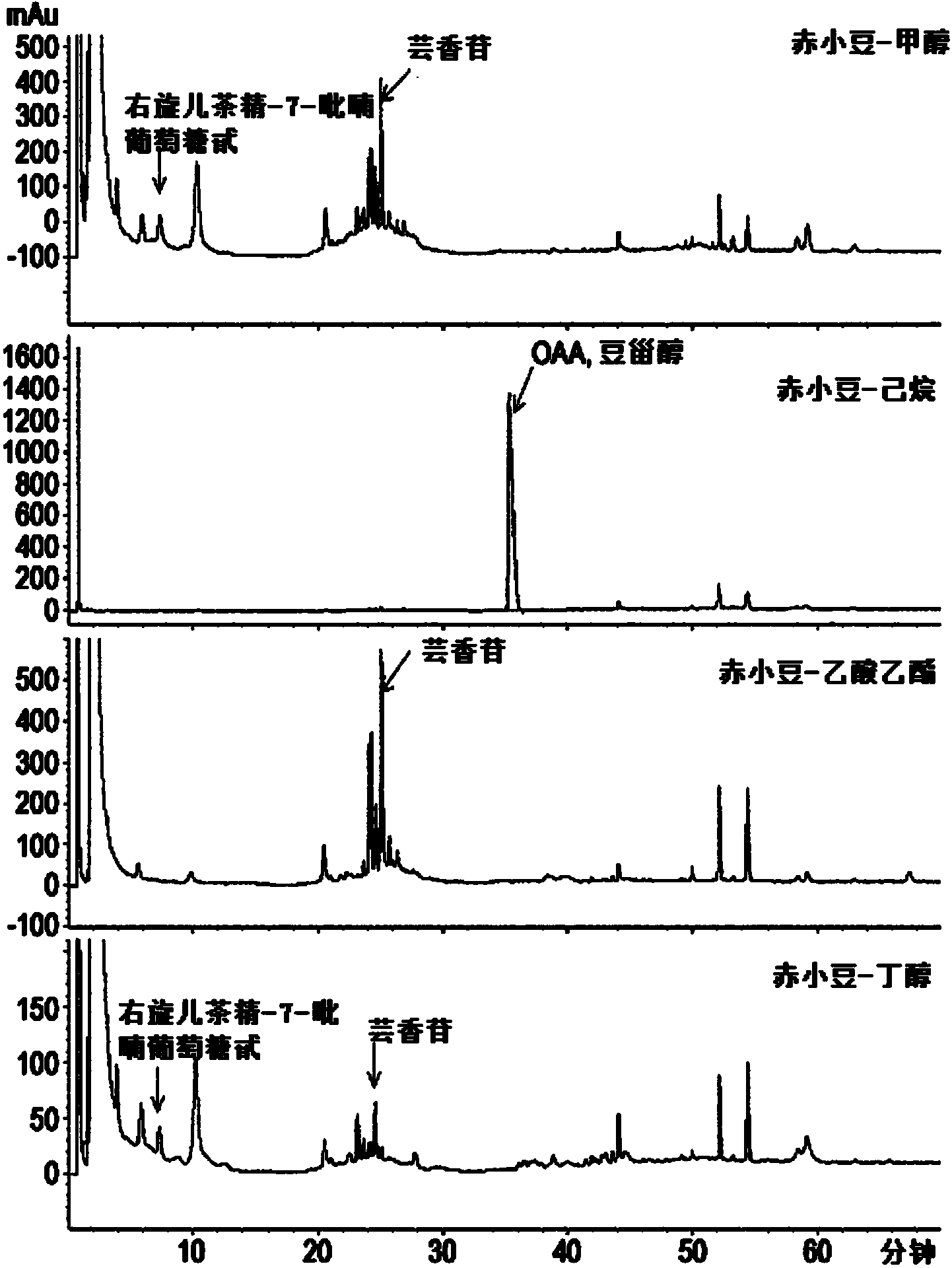
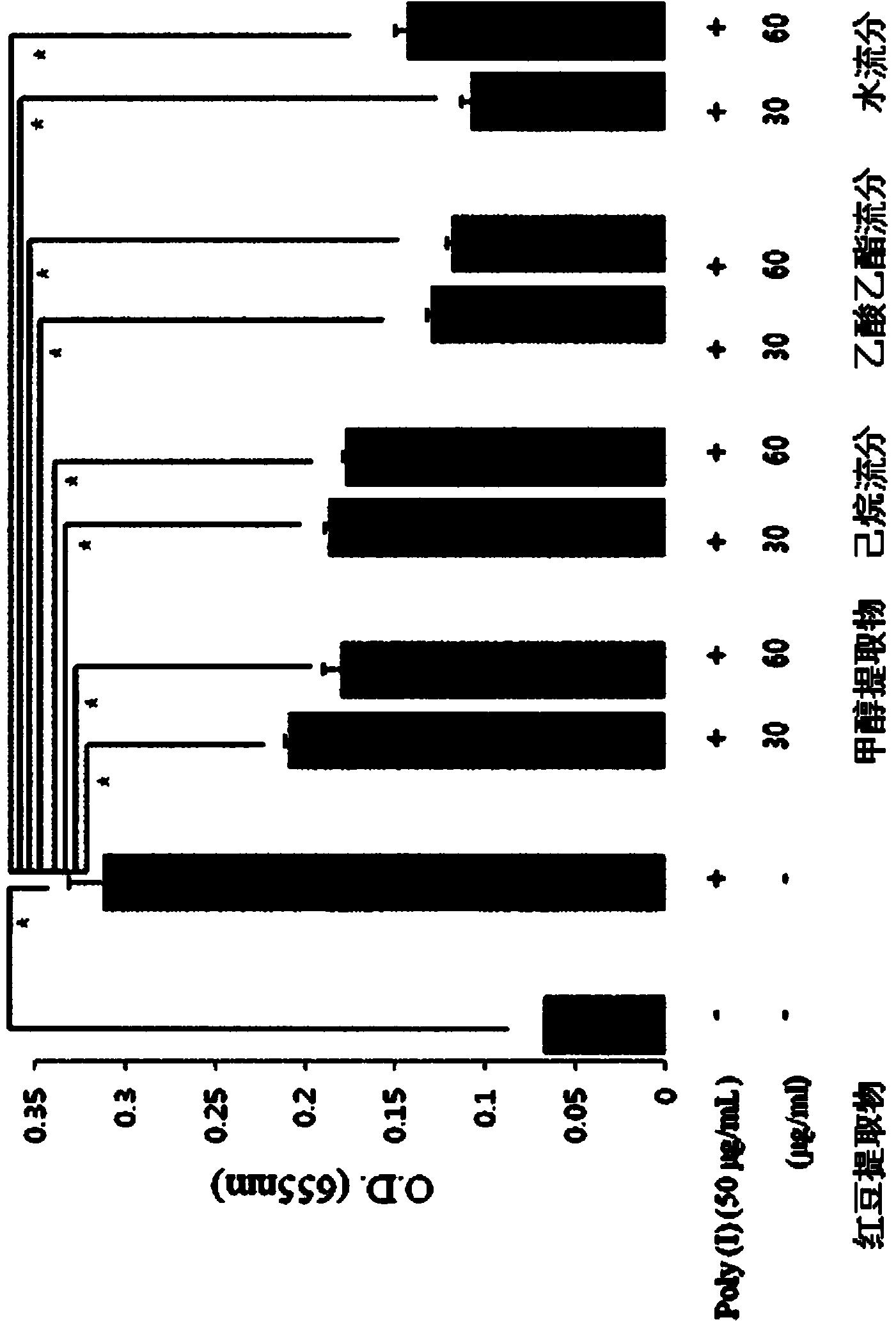
[0092] Example 1: Preparation of Red Bean Extract and Fractions and Compounds of Chemical Formulas 1 and 2 separation and purification of
Embodiment 1-1
[0093] Example 1-1: Preparation of red bean extract
[0094] Wash red beans (red beans or adzuki beans) with water, dry them in a cool place, and grind them into powder using a Waring blender. Add 20 kg of powdered red beans to 100 L of methanol, cold soak and extract for 3 days, filter under reduced pressure with filter paper (Waterman Company, USA), and then use a vacuum rotary concentrator to filter the extract to The methanol solution was removed to obtain 450 g of extraction residue, which is the red bean extract.
Embodiment 1-2
[0095] Example 1-2: Preparation of Fractions
[0096] In order to separate the active fraction from the red bean extract prepared above, after the red bean extract was suspended in 1L of water, an equal amount of n-hexane was added, mixed and graded, and this process was repeated 4 times to obtain 1L of water-soluble Fraction and 4 L of n-hexane soluble fraction.
[0097] Next, the n-hexane-soluble fraction was concentrated under reduced pressure to obtain 50 g of n-hexane-soluble extract.
[0098] In addition, an equal amount of ethyl acetate was added to the 1L water-soluble fraction, mixed and classified, and this process was repeated 3 times, thereby re-obtaining 1L of water-soluble fraction and 3L of ethyl acetate-soluble fraction.
[0099] The obtained ethyl acetate soluble fraction was concentrated under reduced pressure to obtain 35 g of ethyl acetate soluble extract. The remaining water-soluble fraction was concentrated under reduced pressure to 35 g, which was us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com